Chlorination of Nitrobenzene
- Posted by Chemistry instructor
- Categories General
- Date April 3, 2021
Chlorination of Nitrobenzene:
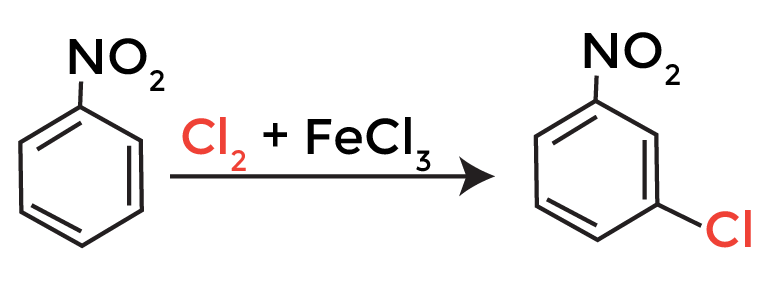
The chlorination of nitrobenzene is an electrophilic substitution reaction, which involves the replacement of a hydrogen atom on the benzene ring with a chlorine atom. The reaction is typically carried out in the presence of a catalyst, such as ferric chloride (FeCl3), and a chlorinating agent, such as chlorine gas (Cl2).
Introduction to Electrophilic Substitution
Electrophilic substitution is a type of organic reaction where an electrophile (an electron-deficient species) replaces a leaving group on a molecule. In the case of aromatic compounds like nitrobenzene, the electrophile attacks the π-electron system of the ring, leading to the formation of a new bond.
Formation of the Electrophile: Chlorinium Ion
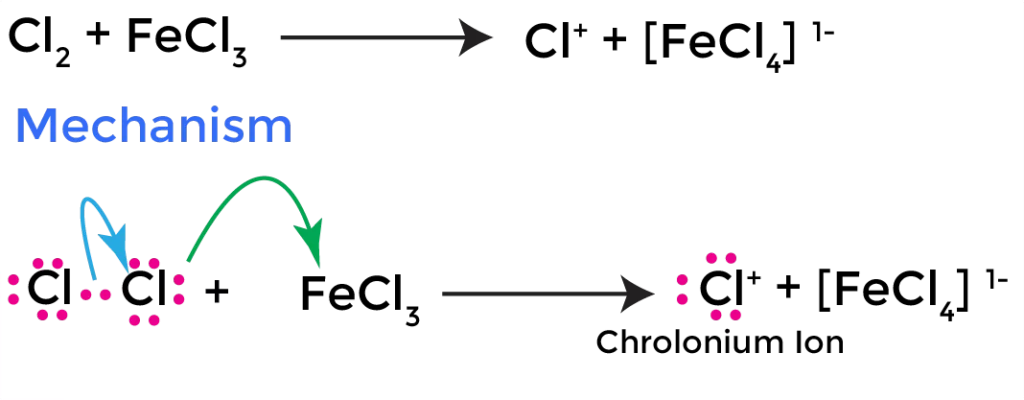
In the chlorination of nitrobenzene, the electrophile is the chlorinium ion (Cl+), which is generated in situ from chlorine gas (Cl2) and a Lewis acid catalyst, such as ferric chloride (FeCl3) or Anhydrous AlCl3.
The chlorinium ion is a strong electrophile, which can attack the π-electron system of the benzene ring.
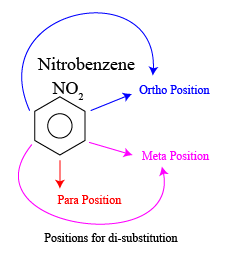
Directing Effect of the Nitro Group
In chlorination of nitrobenzene, the nitro group (-NO2) is a meta-directing group, which means that it directs the incoming electrophile to the meta position (the position at adjacent to the nitro group) on the benzene ring. This is because the nitro group withdraws electron density from the ring, making the meta position more electron-deficient and more susceptible to electrophilic attack.
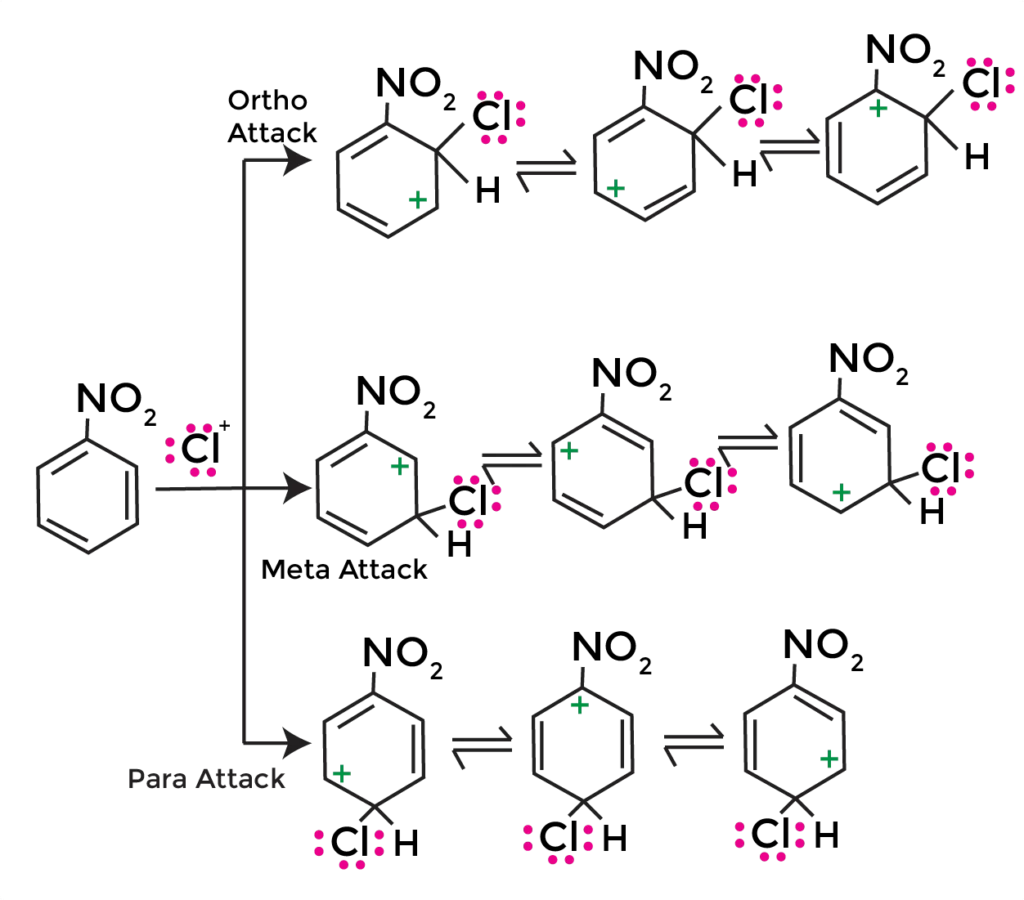
Meta-Substituted Product Formation
In Chlorination of nitrobenzene, when the chlorinium ion attacks the nitrobenzene molecule, it forms a σ-complex, which is a high-energy intermediate. The σ-complex then collapses to form the meta-substituted product, 3-chloronitrobenzene.
The reaction mechanism is as follows:
1. Formation of the chlorinium ion (Cl+)
2. Electrophilic attack by Cl+ on the nitrobenzene molecule, forming a σ-complex
3. Collapse of the σ-complex to form the meta-substituted product, 3-chloronitrobenzene
The resulting product, 3-chloronitrobenzene, is a meta-substituted compound, where the chlorine atom has replaced a hydrogen atom on the benzene ring.
Summary
In summary, the chlorination of nitrobenzene is an electrophilic substitution reaction that involves the formation of a chlorinium ion, which attacks the π-electron system of the benzene ring, leading to the formation of a meta-substituted product, 3-chloronitrobenzene. The nitro group directs the incoming electrophile to the meta position, resulting in the formation of a meta-substituted product.
Tag:CBSE
ABOUT INSTRUCTOR
B.Sc (honors) Chemistry, M.Sc. (Organic Chemistry), Gold Medalist from Gujarat University Ahmedabad. Passionate educator, helping aspirants for IIT-JEE, NEET-UG, AP-Chemistry, IB-HL Chemistry, BIT-SAT, CBSE, and ICSE to achieve their ambitions
You may also like
Analysis of Ammonium Sulphate
Analysis of Ammonium Sulphate Ammonium Sulphate Chemical Composition: Ammonium sulphate, a colorless crystal solid with the chemical formula (NH₄)₂ SO₄, has a rich history and …
Cracking BITSAT: Expert Tips and Strategies to Ace the Exam “Cracking BITSAT: Expert Tips and Strategies to Ace the Exam” – A blog post that …

