Nucleophilic Substitution Reaction bimolecular
SN1 reaction
SN2 Reactions
Play Video: Nucloephilic substitition reaction
Nucleophilic Substitution Reaction bimolecular:
Nucleophilic Substitution Reaction bimolecular is often commonly regarded as an SN2 reaction.
Alkyl Halides React with Nucleophiles because Alkyl halides are polarized at the carbon-halide bond, making the carbon electrophilic
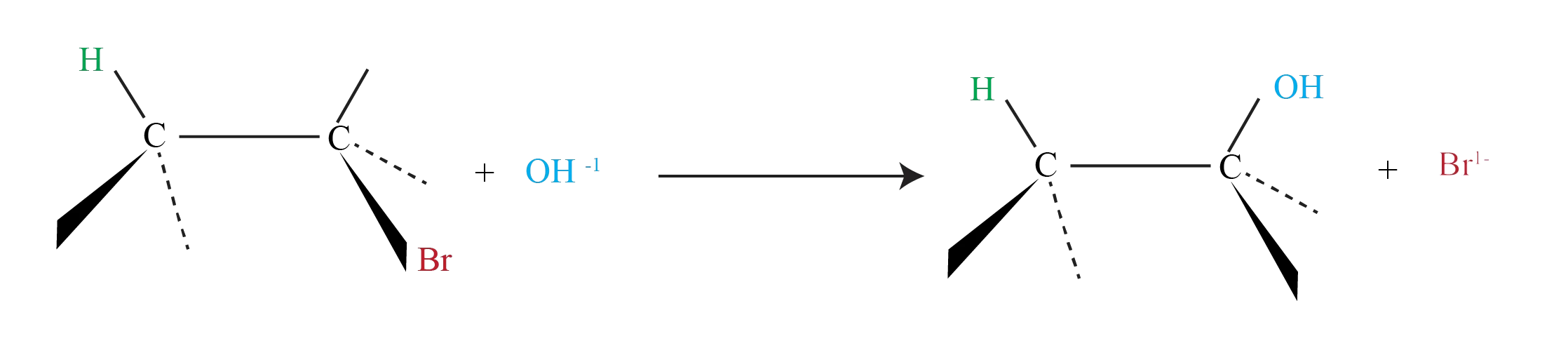
Nucleophiles will replace the halide in C-X bonds of many alkyl halides (reaction as Lewis base)
The Discovery of Nucleophilic Substitution Reactions:
In 1896, Walden showed that (-)-malic acid could be converted to (+)-malic acid by a series of chemical steps with achiral reagents
This established that optical rotation was directly related to chirality and that it changes with chemical alteration
The reaction of (-)-malic acid with PCl5 gives (+)-chloro succinic acid. Further reaction with wet silver oxide gives (+)-malic acid. The reaction series starting with (+) malic acid gives (-) Malic acid
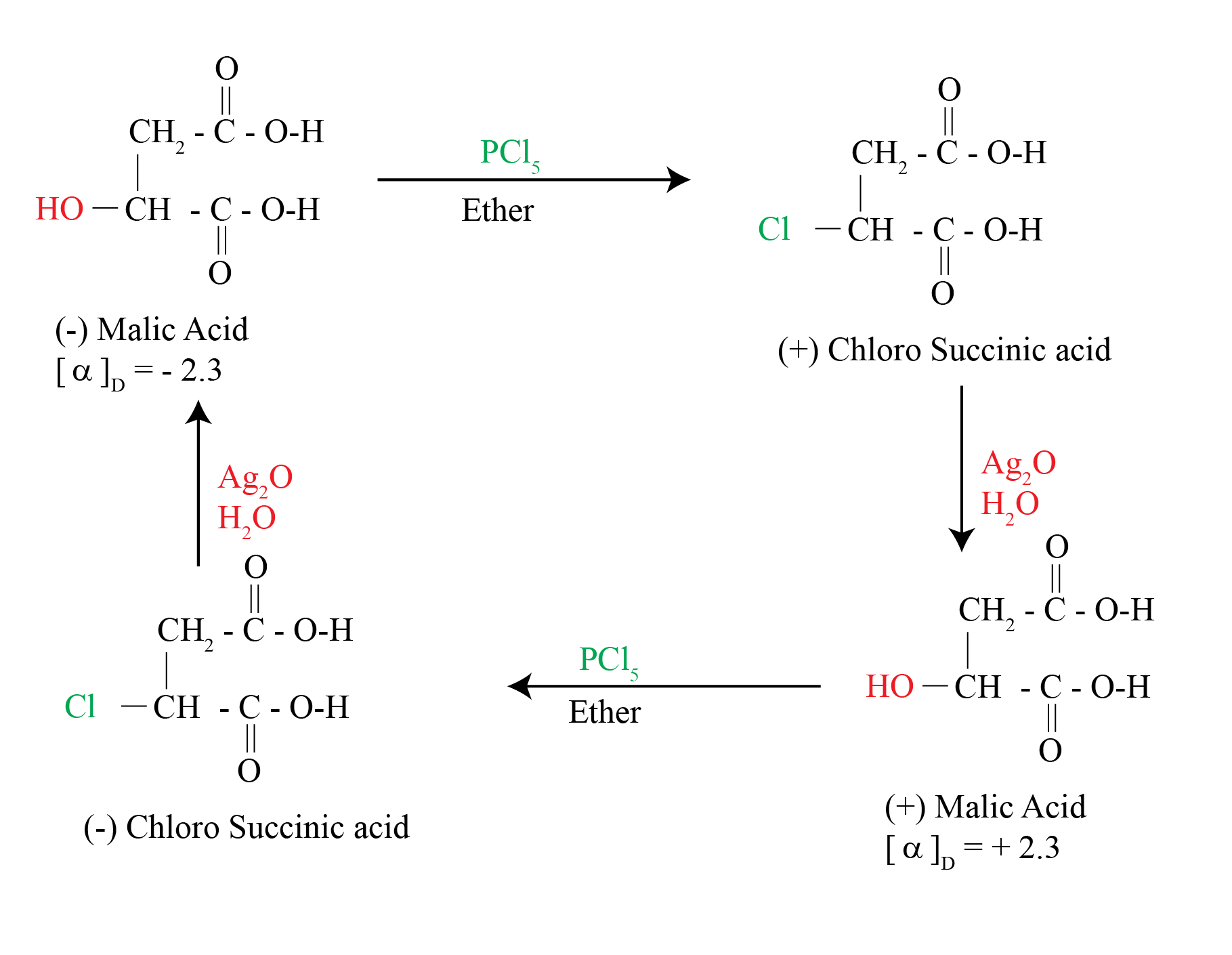
Significance of the Walden Inversion
The reactions alter the array at the chirality center
The reactions involve substitution at that center
Therefore, nucleophilic substitution can invert the configuration at a chirality center.
The SN2 Reaction:
The reaction is with inversion at reacting center
The reaction follows second order reaction kinetics
Ingold nomenclature to describe characteristic step:
S=substitution, N (subscript) = nucleophilic, 2 = both nucleophile and substrate in characteristic step (bimolecular)
SN2 Process
The reaction involves a transition state in which both reactants are together
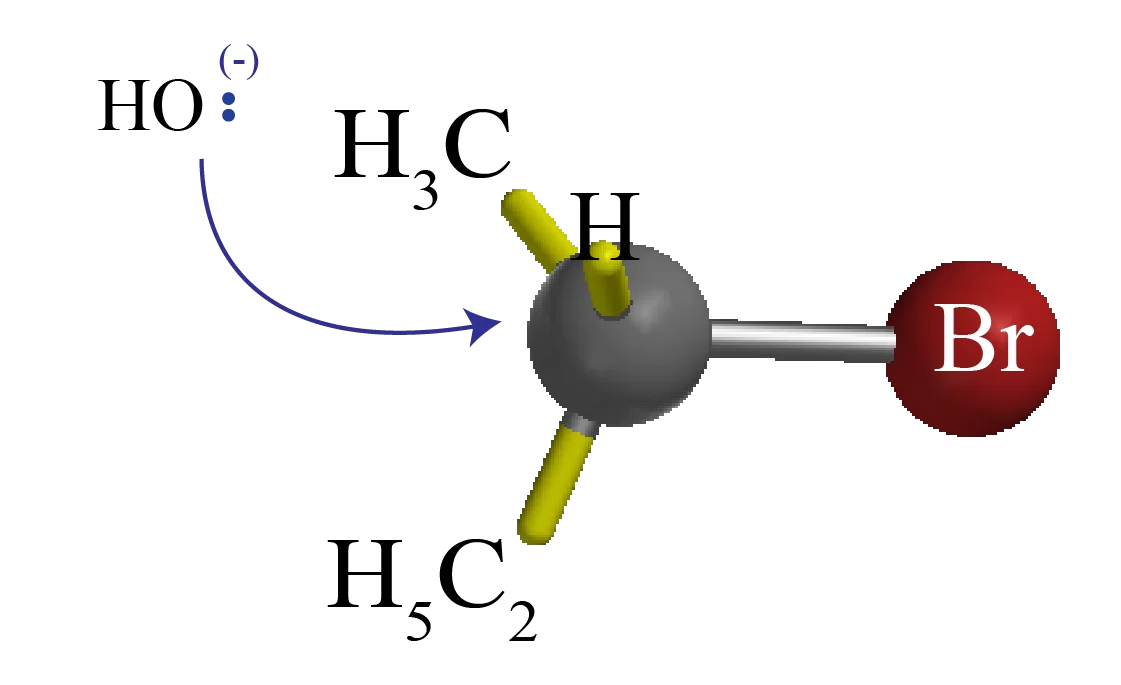
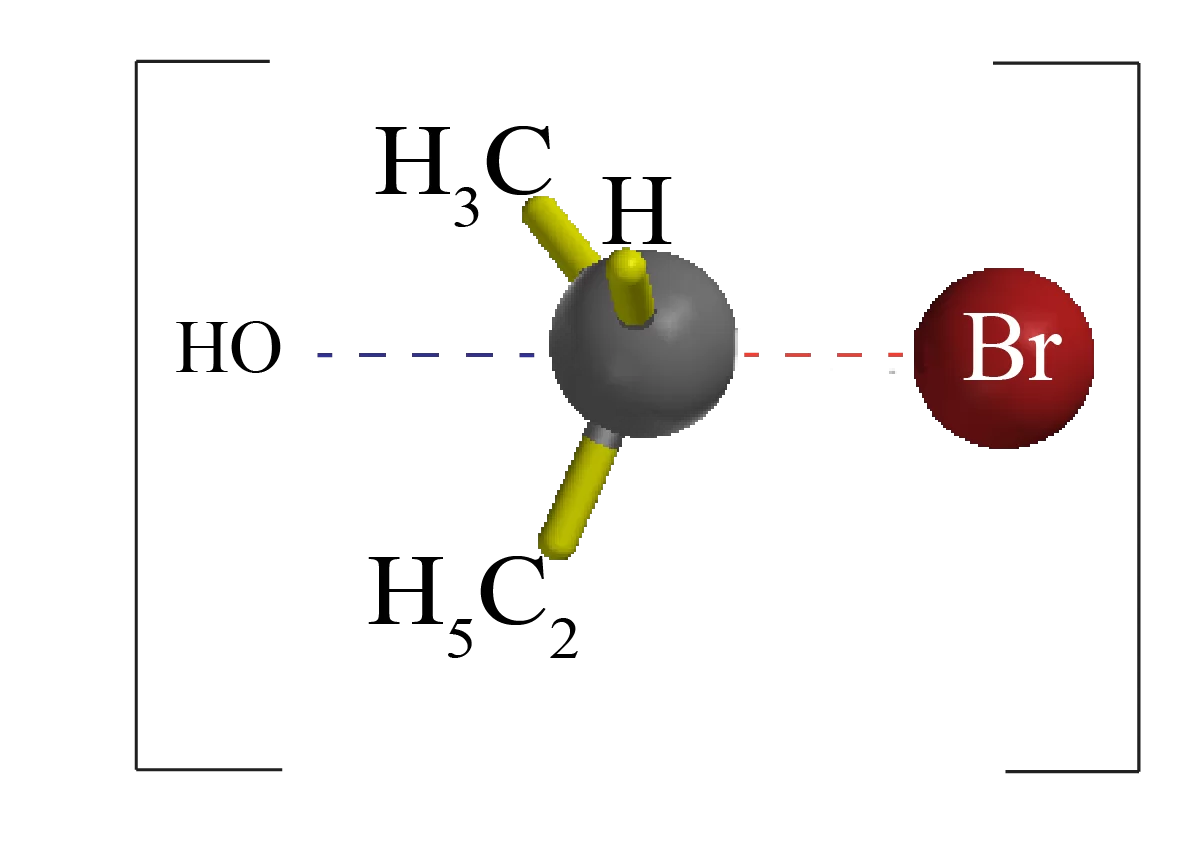

The nucleophile (-OH) uses its lone pair of electrons to attack Alkyl halide carbon 180o away from the leaving halogen. This leads to a transition state with a partially formed C-OH bond and a partially broken C-Br bond.
The stereochemistry of the carbon atom is inverted as the C-OH bond forms fully and the bromide ion leaves with the pair of electrons from the former C-Br bond.
The transition state of an SN2 reaction has a roughly planar arrangement of the carbon atom and the remaining three groups
Characteristics of the SN2 Reaction
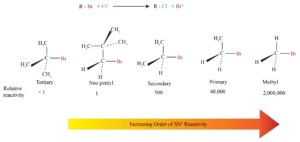
* Sensitive to steric effects
* Methyl halides are the most reactive
* Primary is next most reactive
* Secondary might react
* Tertiary are unreactive by this path
* No reaction at C=C (vinyl halides)
Reactant and Transition State Energy Levels Affect Rate
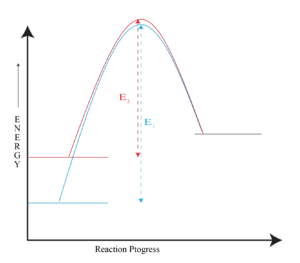
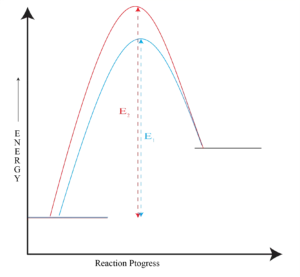
Steric Effects on SN2 Reactions
The carbon atom in (a) bromomethane is readily accessible
resulting in a fast SN2 reaction. The carbon atoms in (b)bromoethane (primary), (c) 2-bromopropane (secondary), and (d) 2-Bromo-2-methylpropane (tertiary) are successively more hindered, resulting in successively slower SN2 reactions.

Order of Reactivity in SN2
The more alkyl groups connected to the reacting carbon, the slower the reaction
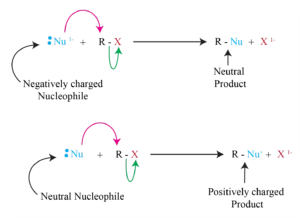
The Nucleophile
* Neutral or negatively charged Lewis base
* Reaction increases coordination at nucleophile
* Neutral nucleophile acquires a positive charge
* Anionic nucleophile becomes neutral
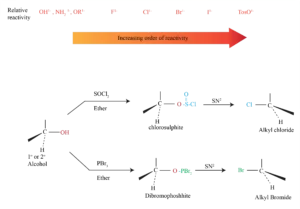
Relative Reactivity of Nucleophiles
* Depends on reaction and conditions
* More basic nucleophiles react faster
* Better nucleophiles are lower in a column of the periodic table
* Anions are usually more reactive than neutrals
* A good leaving group reduces the barrier to a reaction
* Stable anions that are weak bases are usually excellent leaving groups and can delocalize charge.
Poor Leaving Groups:
* If a group is very basic or very small, it prevents reaction
* Alkyl fluorides, alcohols, ethers, and amines do not typically undergo SN2 reactions.
The Solvent
* Solvents that can donate hydrogen bonds (-OH or –NH) slow SN2 reactions by associating with reactants
* Energy is required to break interactions between reactant and solvent
* Polar aprotic solvents (no NH, OH, SH) form weaker interactions with substrate and permit faster reaction.

You May Like:
Tag:CBSE

