How to carry chlorination of benzene
- Posted by Chemistry instructor
- Categories General
- Date February 3, 2022
How to carry chlorination of benzene
Table of Contents

Introduction
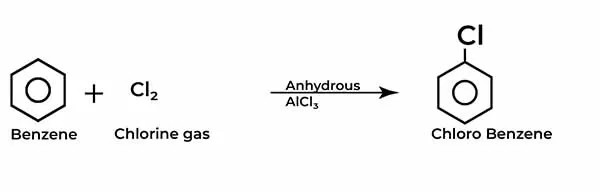
How to carry chlorination of benzene is a fundamental reaction in organic chemistry, representing a crucial step in the synthesis of various aromatic compounds. For students and enthusiasts alike, mastering this process is essential for unlocking the broader world of organic reactions and their applications.
In this blog post, we will delve into the intricacies of How to carry chlorination of benzene, exploring the mechanisms involved, the necessary reagents, and safety precautions, all while providing clear, step-by-step guidance.
Whether you’re preparing for an exam, conducting a laboratory experiment, or simply looking to broaden your chemical knowledge, this comprehensive guide will equip you with the insights and confidence you need to successfully carry out this important reaction.
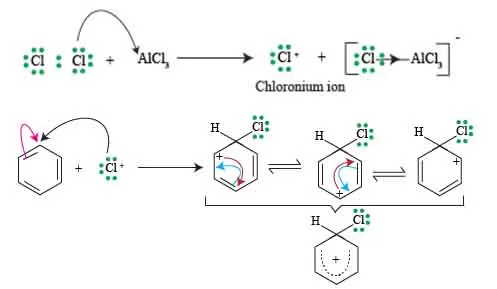
Formation of chlorinium ion leading to sigma complex
In the fascinating world of organic chemistry, the How to carry chlorination of benzene
stands out as a pivotal reaction, showcasing the intricate dance of reactive species and the formation of intermediates.
At the center of this process lies the formation of the chlorinium ion, which plays a crucial role in paving the way to the sigma complex, or arenium ion.
In How to carry chlorination of benzene, benzene reacts with chlorine in the presence of a catalyst, such as iron (III) chloride (FeCl₃) or AlCl3, the first stage of this transformation initiates with the generation of the chlorinium ion.
This ion arises from the interaction between chlorine molecules and the benzene ring, facilitated by the catalytic agent, which polarizes the Cl–Cl bond. As the bond weakens, one of the chlorine atoms carries a positive charge, forming the electrophilic chlorinium ion (Cl⁺). This positively charged ion is highly reactive and seeks to stabilize itself by interacting with the pi-bonding electrons of the benzene molecule.
As the chlorinium ion approaches, it forms a temporary sigma complex, a unique arrangement where the benzene’s aromaticity is momentarily disrupted. In this sigma complex, the bond between one of the carbon atoms in benzene and the chlorinium ion is established, creating a five-membered ring structure that still retains the remaining aromatic character of the other carbon atoms.
Calculating the formation of the chlorinium ion and subsequent sigma complex is a critical step in understanding How to carry chlorination of benzene, the larger mechanism of electrophilic aromatic substitution. This process is not only foundational for chlorination reactions but also demonstrates the delicate balance between stability and reactivity that characterizes many organic transformations.
Thus, exploring these intermediates not only sheds light on chlorination but also enhances our overall appreciation of the underlying principles of organic chemistry.
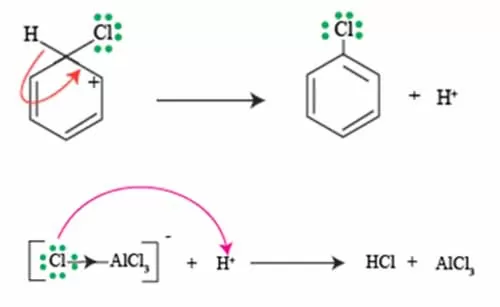
Re-gaining aromaticity from sigma complex
After chlorination, benzene undergoes an electrophilic substitution reaction that forms a sigma complex, often referred to as an arenium ion. This intermediate is crucial because, while it temporarily loses its aromatic stability due to the formation of the sigma bond with the chlorine atom, the restoration of aromaticity is essential for the reaction to proceed effectively.
To regain aromaticity, the system must eliminate a proton from the carbon atom that initially formed the bond with the chlorine. This deprotonation process allows the electron pair from the adjacent carbon to move in, reforming the aromatic pi system. This transformation is typically facilitated by a base, which may be present in the reaction environment or can be a byproduct of the chlorination itself.
In how to carry chlorination of benzene, the loss of the proton and the reestablishment of a stable aromatic ring not only restores the system’s lower energy state but also leads to the final product of the reaction: chlorobenzene.
Understanding this process is critical for students and professionals alike, as it illustrates the delicate balance between stability and reactivity within aromatic compounds. Mastery of this concept enables chemists to manipulate and maximize yields in their synthetic pathways, and platforms like myetutors provide valuable resources and guidance to help deepen this understanding.
In conclusion, we hope this guide on the chlorination of benzene has provided you with a clear and comprehensive understanding of this fundamental reaction in organic chemistry. From the underlying mechanisms to the practical considerations, mastering the chlorination of benzene opens up a world of possibilities in synthetic organic chemistry.
As you continue your studies, remember that platforms like MyEtutors can be invaluable in deepening your understanding and enhancing your skills.
Whether you need further clarification on chlorination or assistance with other topics, don’t hesitate to reach out for help. Embrace your curiosity, experiment confidently, and let your passion for chemistry guide you toward success!
Thank you for reading, and happy studying!
Become a Member
Telegram Channel
YouTube Channel
Tag:CBSE
ABOUT INSTRUCTOR
B.Sc (honors) Chemistry, M.Sc. (Organic Chemistry), Gold Medalist from Gujarat University Ahmedabad. Passionate educator, helping aspirants for IIT-JEE, NEET-UG, AP-Chemistry, IB-HL Chemistry, BIT-SAT, CBSE, and ICSE to achieve their ambitions
You may also like
Analysis of Ammonium Sulphate
Analysis of Ammonium Sulphate Ammonium Sulphate Chemical Composition: Ammonium sulphate, a colorless crystal solid with the chemical formula (NH₄)₂ SO₄, has a rich history and …
Cracking BITSAT: Expert Tips and Strategies to Ace the Exam “Cracking BITSAT: Expert Tips and Strategies to Ace the Exam” – A blog post that …

