How to carry sulphonation of arenes
- Posted by Chemistry instructor
- Categories General
- Date February 11, 2022
How to carry sulphonation of arenes
Table of Contents

Introduction
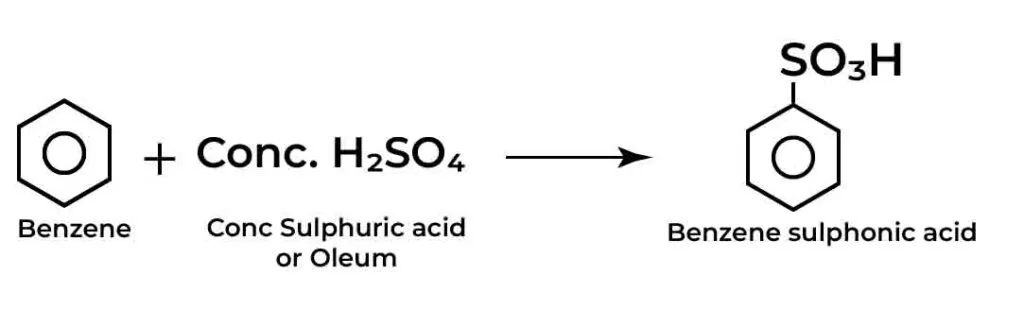
How to carry sulphonation of benzene:
Sulphonation of arenes, particularly benzene, is a fascinating reaction in organic chemistry that opens the door to a wide range of aromatic compounds essential for various applications in pharmaceuticals, dyes, and more.
Understanding this process not only enriches your grasp of electrophilic aromatic substitution reactions but also provides insight into the reactivity and functionalization of aromatic systems.
In this blog post, we’ll delve into the intricacies of sulphonation, outlining key methodologies, reaction mechanisms, and essential tips to help you successfully conduct sulphonation reactions in your laboratory.
Whether you’re a student eager to master the fundamentals or a seasoned chemist looking for a refresher, this comprehensive guide will equip you with the knowledge you need to explore this critical transformation in organic chemistry.
How to carry sulphonation of arenes: Formation of Sulphonium Ion and Sigma Complex
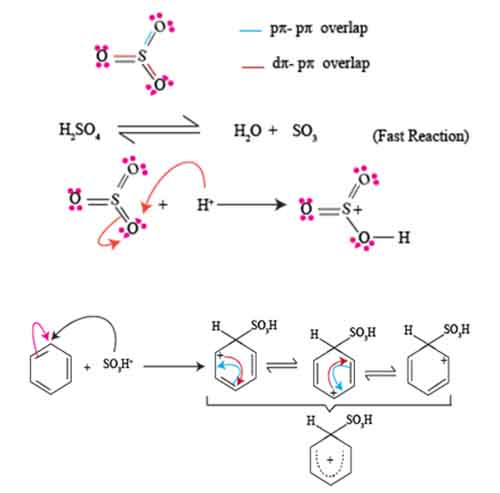
In how to carry sulphonation of arenes, the formation of the sulphonium ion and the sigma complex serves as a pivotal step in directing the overall reaction pathway.
To grasp the significance of this stage, let’s delve into the chemistry behind it. When benzene or another arene reacts with sulfuric acid (H₂SO₄), a potent electrophile is generated: the sulfur trioxide (SO₃) molecule.
how to carry sulphonation of arenes step involves a protonation of the sulfur trioxide to create the highly reactive sulfonium ion (-SO₃H). This sulphonium ion is critical in enhancing the electrophilic character of sulfur, making it an ideal substrate for electrophilic aromatic substitution (EAS).
As the sulphonium ion interacts with the electron-rich arene, it forms a transient sigma complex (also known as the arenium ion). In this complex, the aromatic π system of the benzene ring temporarily loses its aromaticity as one of the double bonds forms a new bond with the sulfur atom.
This leads to the formation of a hexagonal, positively charged intermediate, characterized by resonance stabilization. The electrons from the aromatic ring are crucial here; they help delocalize the positive charge through resonance, providing stability to the sigma complex.
The significance of how to carry sulphonation of arenes process cannot be overstated. The formation of the sulphonium ion allows the arene to undergo the sulphonation reaction more readily, effectively introducing the sulfonyl group (-SO₃H) into the molecule.
Re-gaining aromaticity from sigma complex
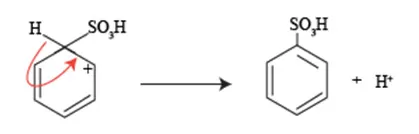
One of the key challenges in how to carry sulphonation of arenes process is to understand how to effectively re-gain the aromaticity that is temporarily lost during the formation of the sigma complex.
The sulphonation reaction begins with the electrophilic attack of sulfuric acid on the aromatic compound, resulting in the creation of a sigma complex (also known as an arenium ion), where the aromatic system is disrupted, and aromaticity is lost. To re-establish aromaticity, the next crucial step involves the loss of a proton from the sigma complex.
In how to carry sulphonation of arenes deprotonation process is essential as it allows the reformation of the aromatic system, restoring the resonance stability that characterizes arenes. Interestingly, the re-gaining of aromaticity in this context is not merely a restoration of structural integrity; it is a key factor that drives the reaction towards completion and ensures the stability of the final sulfonated product.
During this stage, it is important to consider the reaction conditions, such as temperature and the solvent used, as they can significantly influence the efficiency of deprotonation. Utilizing catalytic agents or specific bases can enhance the removal of the proton, facilitating a smoother transition back to the aromatic structure.
Understanding this delicate balance of proton transfer and resonance stability is critical for anyone looking to master the how to carry sulphonation of arenes.
H+ +HSO4 1- → H2SO4 (Spent Acid)
In conclusion, the sulphonation of arenes, particularly benzene, is a fundamental reaction in organic chemistry that opens the door to a myriad of synthetic possibilities.
By understanding the intricacies of this process, from the selection of reagents to the optimal conditions for conducting the reaction, you can significantly enhance your laboratory skills and deepen your comprehension of aromatic chemistry.
We hope this guide has illuminated the steps involved and provided you with the confidence to tackle sulphonation in your own experiments.
For further learning and personalized assistance, consider exploring myetutors, where expert guidance is just a click away. Embrace the challenges of organic chemistry, and watch your skills flourish!
Become a Member
Telegram Channel
YouTube Channel
Tag:CBSE
ABOUT INSTRUCTOR
B.Sc (honors) Chemistry, M.Sc. (Organic Chemistry), Gold Medalist from Gujarat University Ahmedabad. Passionate educator, helping aspirants for IIT-JEE, NEET-UG, AP-Chemistry, IB-HL Chemistry, BIT-SAT, CBSE, and ICSE to achieve their ambitions
You may also like
Analysis of Ammonium Sulphate
Analysis of Ammonium Sulphate Ammonium Sulphate Chemical Composition: Ammonium sulphate, a colorless crystal solid with the chemical formula (NH₄)₂ SO₄, has a rich history and …
Cracking BITSAT: Expert Tips and Strategies to Ace the Exam “Cracking BITSAT: Expert Tips and Strategies to Ace the Exam” – A blog post that …

