Friedel craft alkylation reaction
- Posted by Chemistry instructor
- Categories Blog
- Date February 17, 2022
Friedel craft alkylation reaction
Table of Contents

Introduction
The world of organic chemistry offers a fascinating array of reactions that unlock a myriad of possibilities for synthesizing complex compounds. One such reaction, the Friedel craft alkylation reaction, stands out for its ability to introduce alkyl groups onto aromatic rings, making it a cornerstone of electrophilic substitution reactions.
If you’re a chemistry enthusiast or a student eager to deepen your understanding, this blog post will delve into the intricate details of the Friedel craft alkylation reaction, exploring its mechanism, applications, and potential pitfalls.
Join us as we unravel this essential reaction that not only enriches your knowledge but also enhances your practical skills in the lab with the help of myetutors.
Friedel craft alkylation reaction formation of carbonium ion
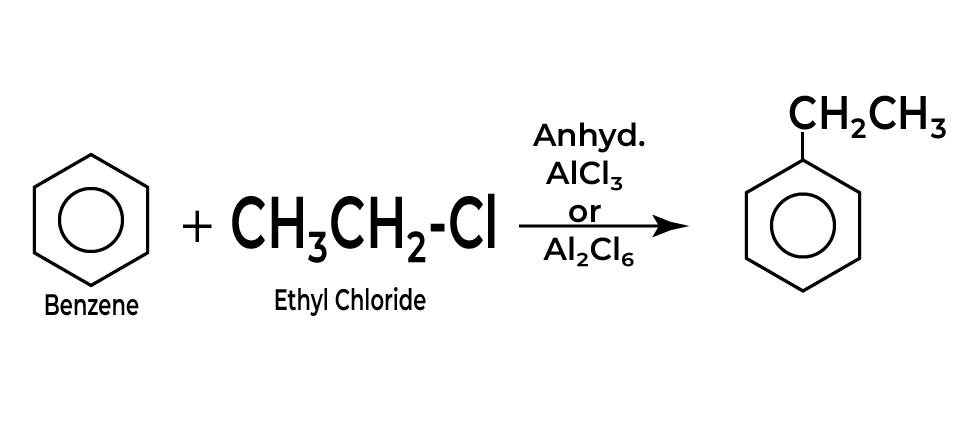
At the heart of the Friedel craft alkylation reaction lies the crucial step of carbonium ion formation, a pivotal process in the electrophilic substitution mechanism.
To begin with, the alkyl halide, which serves as our alkylating agent, reacts with a Lewis acid catalyst commonly aluminum chloride (AlCl₃). This interaction is essential, as the Lewis acid facilitates the generation of the reactive electrophile.
As the alkyl halide interacts with the Lewis acid, the halogen is effectively removed, generating a positively charged species known as a carbonium ion (or carbocation). This ion is typically stabilized by the surrounding electron-rich aromatic system, contributing to the overall stability of the resulting complex.
The structure of the carbonium ion varies depending on the nature of the alkyl group designated for substitution; for example, tertiary carbonium ions are more stable due to hyperconjugation and inductive effects compared to secondary or primary ones.
Once formed, the carbonium ion possesses a strong electrophilic character, making it highly reactive and ready to engage in subsequent steps of the Friedel craft alkylation reaction.
This reactivity allows the carbonium ion to attack the π electrons of benzene, facilitating the formation of the σ-complex.
Understanding how carbonium ions are formed and their role in the Friedel craft alkylation reaction mechanism is fundamental for mastering this electrophilic substitution reaction
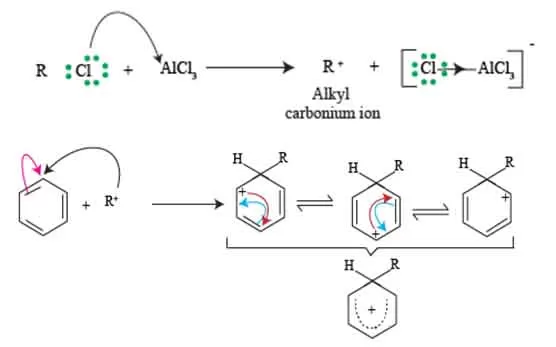
When a benzene molecule undergoes an electrophilic substitution reaction, it forms an intermediate known as the sigma complex, or arenium ion. This species is generated when the electrophile attacks the aromatic ring, temporarily disrupting the aromaticity of benzene and forming a covalent bond between the electrophile and one of the carbon atoms in the ring.
The sigma complex is characterized by its positive charge, which induces a state of instability. However, this is where resonance comes into play. The unstable nature of the sigma complex allows for resonance stabilization, where the positive charge can be delocalized across the aromatic system. This delocalization occurs through the rearrangement of pi electrons, allowing the positive charge to resonate among different carbon atoms of the benzene ring.
As a result, the complex can be represented by multiple resonance structures, each depicting the positive charge located on a different carbon atom. This resonance not only stabilizes the intermediate but also enhances its reactivity, making it more likely to undergo deprotonation, reverting back to a stable aromatic compound.
Understanding the resonance of the sigma complex is essential, as the ease with which the positive charge is delocalized influences the overall reaction kinetics and the likelihood of achieving a successful alkylation product.
Thus, comprehending the resonance mechanics involved in the sigma complex underscores its pivotal role in the Friedel craft alkylation reaction.
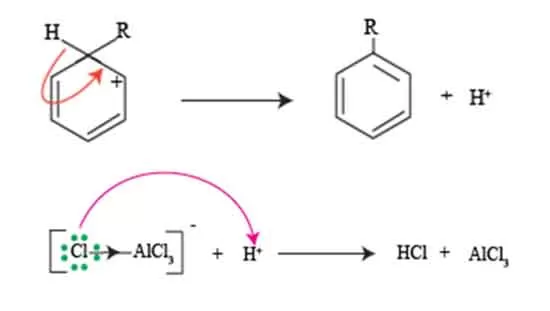
Regaining aromaticity of sigma complex
This intermediate, characterized by the temporary loss of benzene’s aromatic stability, is often less stable than the original aromatic ring due to the sp3 hybridization of the carbon now bound to the electrophile.
Consequently, one of the key objectives in the Friedel craft alkylation reaction is to swiftly regenerate the aromaticity of the ring. The restoration of aromaticity occurs in two significant steps: first, the loss of a proton from the carbon atom that was initially bonded to the electrophile, and second, the reformation of the π-electron system.
This proton loss is often facilitated by the presence of a base, which helps stabilize the reaction by accepting the proton and allowing the electrons to regain their delocalized state. The result is a reformed aromatic compound, now substituted with the alkyl group a successful transformation that beautifully exemplifies the intricacies of electrophilic substitution reactions.
Understanding this regaining of aromaticity not only underscores the elegance of Friedel craft alkylation reaction but also enriches your grasp of foundational organic chemistry principles.
Become a Member
YouTube Channel
Telegram Channel
Tag:CBSE
ABOUT INSTRUCTOR
B.Sc (honors) Chemistry, M.Sc. (Organic Chemistry), Gold Medalist from Gujarat University Ahmedabad. Passionate educator, helping aspirants for IIT-JEE, NEET-UG, AP-Chemistry, IB-HL Chemistry, BIT-SAT, CBSE, and ICSE to achieve their ambitions
You may also like
Acid catalyzed dehydration of alcohols
Acid catalyzed dehydration of alcohols Cracking Organic Chemistry: Top 5 Tips to Master Acid Catalyzed Dehydration of Alcohols. A blog post that provides valuable insights …
AP Chemistry Information
AP Chemistry Information Table of Contents: About Advance Placement Program AP Chemistry Overview AP Course Content AP Chemistry Practical AP Exam Assessment About the Advanced …
How to crack NEET UG 2025
How to crack NEET UG 2025 How to crack NEET UG 2025 How to prepare for NEET UG in 6 months The NEET (UG) is …
