How to carry nitration of arenes
- Posted by Chemistry instructor
- Categories General
- Date February 8, 2022
How to carry nitration of arenes
Table of Contents


How to carry nitration of arenes introduction
Navigating the intricacies of organic chemistry can often feel like uncharted territory, especially when it comes to complex reactions such as the how to carry nitration of arenes, including benzene.
This crucial process not only has significant implications in synthetic chemistry but also plays a pivotal role in the production of various important chemicals and pharmaceuticals.
Whether you’re a student striving to master this essential concept or a seasoned chemist looking to refresh your knowledge, understanding the mechanisms and conditions necessary for effective nitration is key.
In this blog post, we will explore the step-by-step procedures, safety precautions, and practical tips to help you successfully carry out the how to carry nitration of arenes,
Formation of carbonium ion and sigma complex
In how to carry nitration of arenes, a pivotal step is the formation of the carbonium ion and the sigma complex, which are crucial intermediates in this electrophilic aromatic substitution reaction.
in how to carry nitration of arenes, process begins when an electrophile, typically the nitronium ion (NO₂⁺), generated from a mix of concentrated nitric acid and sulfuric acid, approaches the aromatic ring.
The unique stability of the aromatic system plays a significant role here, as the electrons in the π system of the benzene ring interact with the electron-deficient nitronium ion. As the reaction unfolds, one of the carbon atoms in the ring donates a pair of electrons to form a bond with the nitronium ion.
This leads to the creation of a sigma complex, also known as an arenium ion or Wheland intermediate. In how to carry nitration of arenes transient structure, the aromaticity of the benzene ring is temporarily lost, resulting in a positively charged carbonium ion. The formation of the sigma complex is a reversible reaction, characterized by an intermediate state where the aromatic system is no longer fully conjugated.
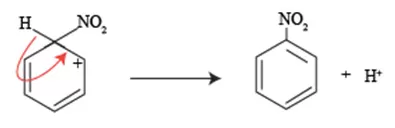
The carbonium ion is inherently unstable and can undergo deprotonation to restore aromaticity. A base, often the bisulfate ion (HSO₄⁻) formed during the nitration process, abstracts a proton from one of the carbon atoms bonded to the nitronium ion, regenerating the aromaticity of the ring and yielding the final nitro-substituted product, nitro-arene.
Understanding the dynamics of carbonium ion and sigma complex formation provides insight into the mechanisms of nitration reactions. It highlights the balance between electrophilic attack and the need to restore aromatic stability, marking crucial steps that chemistry students need to master when studying nitration of arenes with resources .

Re-generation of Aromaticity from sigma complex
In how to carry nitration of arenes, one of the most fascinating transformations occurs during the re-generation of aromaticity from the sigma complex. This critical step not only illustrates the delicate balance between electrophilic substitution and resonance stabilization but also highlights the nuanced mechanisms at play in organic chemistry.
When arenes react with nitrating agents, typically a mixture of concentrated nitric acid (HNO3) and sulfuric acid (H2SO4), they initially form an arenium ion, often referred to as the sigma complex or Wheland intermediate.
In this transient state, the aromatic system loses its distinctive aromaticity due to the introduction of a nitro group (NO2) into one of the carbon atoms, creating a positively charged intermediate.
As we delve deeper into this process, it is essential to understand how the re-establishment of aromatic character occurs. The sigma complex is inherently unstable, and the re-generation of aromaticity transpires through a series of resonance structures that delocalize the positive charge. Essentially, the electron-withdrawing nature of the nitro group creates a favorable driving force for the loss of a proton, restoring the aromatic stability of the original benzene ring.
This regeneration is achieved when the sigma complex undergoes a proton loss from the carbon atom that was initially bonded to the electrophile, leading to a return to the aromatic state. The product of this reaction is a nitroarene, where the aromatic system is fully restored, showcasing the profound resilience of benzene’s structure despite the disruption caused by electrophilic attack.
Understanding this mechanism is not only crucial for organic chemists but also enriches our grasp of reaction dynamics and the enduring significance of aromaticity in organic compounds.
In conclusion, mastering the process of how to carry nitration of arenes and benzene is an essential skill for any aspiring organic chemistry student. By understanding the underlying principles, optimal conditions, and safety precautions outlined in this post, you can confidently approach your nitration experiments with the knowledge needed to achieve successful outcomes.
Whether you’re preparing for an exam or looking to enhance your lab skills, the insights provided here are aimed at demystifying this important reaction. If you’re seeking further guidance or personalized tutoring on organic chemistry topics, myetutors is here to support your learning journey.
Thank you for reading, and we look forward to helping you excel in your studies!
Become a Member
Telegram Channel
YouTube Channel
Tag:CBSE
ABOUT INSTRUCTOR
B.Sc (honors) Chemistry, M.Sc. (Organic Chemistry), Gold Medalist from Gujarat University Ahmedabad. Passionate educator, helping aspirants for IIT-JEE, NEET-UG, AP-Chemistry, IB-HL Chemistry, BIT-SAT, CBSE, and ICSE to achieve their ambitions
You may also like
Analysis of Ammonium Sulphate
Analysis of Ammonium Sulphate Ammonium Sulphate Chemical Composition: Ammonium sulphate, a colorless crystal solid with the chemical formula (NH₄)₂ SO₄, has a rich history and …
Cracking BITSAT: Expert Tips and Strategies to Ace the Exam “Cracking BITSAT: Expert Tips and Strategies to Ace the Exam” – A blog post that …

