Curriculum
- 12 Sections
- 69 Lessons
- 100 Hours
- Chapter 1 (Solid State)4
- Chapter 2 (Solutions)10
- 2.0Types of solutions20 Minutes
- 2.1Ways of expressing concentrations 120 Minutes
- 2.2Ways of expressing concentrations 220 Minutes
- 2.3Henry’s Law and applications 120 Minutes
- 2.4Raoult’s Law and applications 220 Minutes
- 2.5Colligative property 110 Minutes
- 2.6Colligative property 215 Minutes
- 2.7Colligative property 315 Minutes
- 2.8Colligative property 415 Minutes
- 2.9Abnormal Molecular Mass15 Minutes
- Chapter 3 (Electro Chemistry)8
- 3.1Electrolytic conductivity 120 Minutes
- 3.2Debye Huckel limiting law15 Minutes
- 3.3Electrochemical Cell20 Minutes
- 3.4Cell potential in electrochemistry20 Minutes
- 3.5How to use Nernst equation20 Minutes
- 3.6Gibbs free energy in electrochemistry20 Minutes
- 3.7Understand Faradays Laws of Electrolysis20 Minutes
- 3.8Fundamentals of commercial batteries20 Minutes
- Chapter 4 (Chemical Kinetics)6
- 4.1How to calculate rate of reaction20 Minutes
- 4.2How to calculate reaction law and orders20 Minutes
- 4.3Initial rate method know it all faster15 Minutes
- 4.4How to use integral method in kinetics 120 Minutes
- 4.5How to use integral method in kinetics 215 Minutes
- 4.6How Collision Theory Explains Chemical Reactions20 Minutes
- Chapter 8 (d-block Elements)7
- 5.1d block electron configuration charts10 Minutes
- 5.2Trend across d block 1 atomic radii10 Minutes
- 5.3Trend across d block 2 melting point10 Minutes
- 5.4Trend across d block 3 Ionization energy10 Minutes
- 5.5Trend across d block 4 Oxidation state10 Minutes
- 5.6Trend in d block 5 Electrode Potential10 Minutes
- 5.7Trend in d block metal properties
- Chapter 9 (Co-ordinate Compounds)6
- Chapter 10 (Haloalkanes Haloarenes)7
- Chapter 11 (Alcohols, Phenols Ethers)7
- Chapter 12 (Aldehyde Ketones)4
- Chapter 12 (Carboxylic acid, Derivatives)3
- Chapter 13 (Amines)3
- Chapter 14 (Biomolecules)4
Cell potential in electrochemistry
Cell potential in electrochemistry
Table of Contents

Introduction
Understanding Cell potential in electrochemistry is a pivotal aspect of electrochemistry that can unlock the mysteries behind chemical reactions and their energetic outcomes. In this insightful blog post, we delve into the intricacies of cell potential, exploring its fundamental principles and applications.
Whether you’re a student grappling with the complexities of electrochemical cells or a seasoned chemistry enthusiast seeking to enhance your knowledge, grasping how to calculate standard cell potential is essential. We’ll guide you through the theoretical underpinnings, practical calculations, and how to leverage this knowledge to predict reaction feasibility
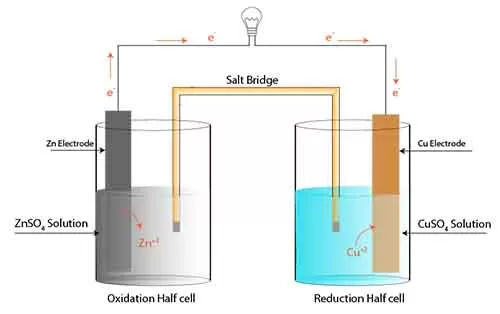
Cell potential in electrochemistry : Cell representation
Understanding cell representation is crucial for grasping how electrochemical cells function and how to interpret cell potentials effectively for Cell potential in electrochemistry.
Cell representation follows a standardized notation known as the cell notation or line notation, which provides a concise way to describe the components and reactions occurring within an electrochemical cell.
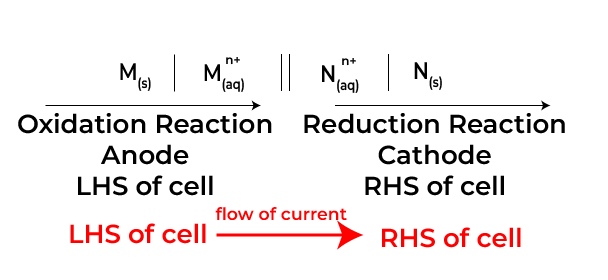
A typical Cell potential in electrochemistry can be represented as a two-part setup, consisting of an anode and a cathode, each involving a half-reaction. The cell notation displays these two components separated by a double vertical line (||), which signifies the salt bridge or porous barrier allowing ion transfer while preventing direct mixing of different solutions.
For example, a common representation may look like this:
Zn | Zn²⁺ (aq) || Cu²⁺ (aq) | Cu. Here, the left side indicates the anode (or oxidation Reaction—where oxidation occurs) showing solid zinc (Zn) in contact with zinc ions in solution (Zn²⁺). The right side highlights the cathode( or reduction reaction) where reduction takes place with copper ions in solution (Cu²⁺) next to solid copper (Cu).
The direction of electron flow is also an essential aspect of cell representation. Electrons move from the anode to the cathode, which can be inferred from the defined roles of oxidation and reduction occurring at each electrode. Cell potential in electrochemistry clear delineation helps in predicting the spontaneous nature of reactions, allowing chemists to calculate the standard cell potential (E°) using standard reduction potentials from a table of reduction half-reactions.
How to Calculate Standard Cell Potential
Understanding how to calculate standard cell potential is a fundamental skill in Cell potential in electrochemistry, enabling students and professionals alike to determine the feasibility of redox reactions. The standard cell potential (E°cell) provides insight into the tendency of a chemical reaction to occur spontaneously.
To calculate it, you need the standard reduction potentials of the half-reactions involved in your electrochemical cell. First, gather the standard reduction potentials from a reliable table, which usually lists these values at standard conditions (25°C and 1 atm). The standard reduction potential for a half-reaction indicates how easily a species gains electrons; the more positive the value, the stronger the oxidizing agent.
To calculate the standard cell potential, follow these simple steps:
1.Identify the Half-Reactions: Write out the oxidation and reduction half-reactions for the electrochemical reaction you are studying.
2.Use the Standard Reduction Potentials: Look up the standard reduction potentials (E°) for each half-reaction. Remember that the reduction half-reaction retains its original potential, while the oxidation half-reaction’s potential will need to be reversed.
3. Calculate E°cell: The equation to calculate the standard cell potential is given below. This means you subtract the oxidation potential from the reduction potential.
4. Interpret the Result: If E°cell is positive, the reaction is spontaneous under standard conditions. Conversely, a negative E°cell indicates that the reaction is non-spontaneous. By mastering the calculation of standard cell potential, you will gain deeper insight into the behavior of electrochemical cells, paving the way for a better understanding of important concepts in electrochemistry, such as electrolysis, battery functionality, and corrosion processes.

Numerical
A cell is prepared by dipping a copper rod in 1 M CuSO4 aqueous and a Nickel rod in 1M NiSO4 aqueous solution. The Standard reduction potentials for Cu-electrode and Ni-electrode are +0.34 and -0.24 Volt respectively Calculate
- What will be the cel reaction?
- What will be the standard emf of the cell?
- Which electrode will be positive?
- How will the cell be represented?
- Which electrode is anode?
Given:
Standard Reduction potential
Cu → + 0.34 V; Ni → – 0.24 V
Solution:
Nickel has negative reduction potential so it will oxidize, and Cu will get reduced
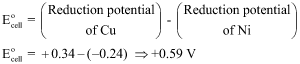
EMF of the cell is +0.59 V
The overall cell reaction is
Ni(s) + CuSO4 → NiSO4 + Cu(s)
The Cu electrode is positive electrode as reduction is taking place at Cathode.
The Cell representation is
Ni(s) | NiSO4 (aq) || CuSO4 (aq) | Cu(s)
Ni electrode is anode
Calculating the standard cell potential is a fundamental skill in electrochemistry, allowing you to predict the feasibility of redox reactions and assess the energy that can be obtained from electrochemical cells. The standard cell potential (E°) is determined using the standard reduction potentials of the half-reactions involved in the electrochemical process, which can be found in electrochemical tables.
To start, it’s crucial to write the relevant half-reactions for the oxidation and reduction processes. The reduction half-reaction occurs at the cathode, while the oxidation half-reaction takes place at the anode. Next, you will look up the standard reduction potential (E°) values for each half-reaction from these tables, which are typically measured against the standard hydrogen electrode (SHE).
Once you have these values, simply follow this formula to calculate the standard cell potential:

In this equation, the E° for the reduction half-reaction should remain unchanged, while you must negate the E° value for the oxidation half-reaction since it demonstrates a loss of electrons.
The standard cell potential combines the tendencies of the oxidized and reduced forms to gain and lose electrons, ultimately giving you a quantifiable measure of cell potential under standard conditions (1 M concentration, 1 atm pressure, and a temperature of 25°C).
A positive standard cell potential indicates a spontaneous reaction, while a negative value suggests non-spontaneity.
