Curriculum
- 12 Sections
- 69 Lessons
- 100 Hours
- Chapter 1 (Solid State)4
- Chapter 2 (Solutions)10
- 2.0Types of solutions20 Minutes
- 2.1Ways of expressing concentrations 120 Minutes
- 2.2Ways of expressing concentrations 220 Minutes
- 2.3Henry’s Law and applications 120 Minutes
- 2.4Raoult’s Law and applications 220 Minutes
- 2.5Colligative property 110 Minutes
- 2.6Colligative property 215 Minutes
- 2.7Colligative property 315 Minutes
- 2.8Colligative property 415 Minutes
- 2.9Abnormal Molecular Mass15 Minutes
- Chapter 3 (Electro Chemistry)8
- 3.1Electrolytic conductivity 120 Minutes
- 3.2Debye Huckel limiting law15 Minutes
- 3.3Electrochemical Cell20 Minutes
- 3.4Cell potential in electrochemistry20 Minutes
- 3.5How to use Nernst equation20 Minutes
- 3.6Gibbs free energy in electrochemistry20 Minutes
- 3.7Understand Faradays Laws of Electrolysis20 Minutes
- 3.8Fundamentals of commercial batteries20 Minutes
- Chapter 4 (Chemical Kinetics)6
- 4.1How to calculate rate of reaction20 Minutes
- 4.2How to calculate reaction law and orders20 Minutes
- 4.3Initial rate method know it all faster15 Minutes
- 4.4How to use integral method in kinetics 120 Minutes
- 4.5How to use integral method in kinetics 215 Minutes
- 4.6How Collision Theory Explains Chemical Reactions20 Minutes
- Chapter 8 (d-block Elements)7
- 5.1d block electron configuration charts10 Minutes
- 5.2Trend across d block 1 atomic radii10 Minutes
- 5.3Trend across d block 2 melting point10 Minutes
- 5.4Trend across d block 3 Ionization energy10 Minutes
- 5.5Trend across d block 4 Oxidation state10 Minutes
- 5.6Trend in d block 5 Electrode Potential10 Minutes
- 5.7Trend in d block metal properties
- Chapter 9 (Co-ordinate Compounds)6
- Chapter 10 (Haloalkanes Haloarenes)7
- Chapter 11 (Alcohols, Phenols Ethers)7
- Chapter 12 (Aldehyde Ketones)4
- Chapter 12 (Carboxylic acid, Derivatives)3
- Chapter 13 (Amines)3
- Chapter 14 (Biomolecules)4
d block electron configuration charts
d block electron configuration charts
Table of Contents

Introduction
Understanding d block electron configuration charts is crucial for mastering chemistry, especially when it comes to the complex world of d block elements. These transition metals, known for their unique properties and behaviors, can often leave students feeling overwhelmed when it comes to grasping their electron arrangements.
In this blog post d block electron configuration charts, we’ll explore the significance of d block electron configuration charts, breaking down how to read and interpret them effectively.
Whether you’re a student looking to improve your grades or simply a curious learner, you’ll find essential insights and tips that will make navigating the intricacies of d block elements a breeze. Dive in as we unravel the secrets to understanding these fascinating elements and empower your learning journey!
Definition
Transition metals are a unique group of elements that share distinct properties and characteristics, making them essential in both chemistry and various practical applications.
Located in the d-block of the periodic table, transition metals consist of elements that have partially filled d orbitals. This group includes well-known elements such as iron, copper, and nickel, among others.
What sets transition metals apart from other metal groups is their ability to form multiple oxidation states. This means they can lose different numbers of electrons during chemical reactions, leading to a variety of chemical behaviors and the formation of a myriad of compounds.
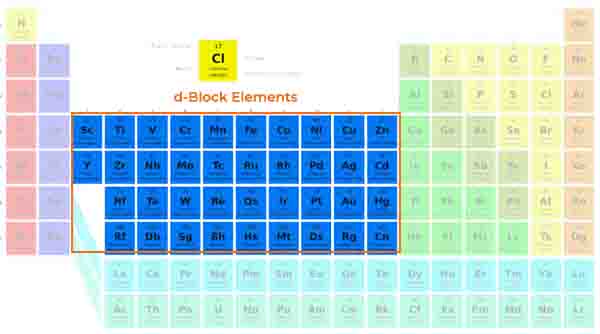
Electronic Configuration
The general electronic configuration of d-Block elements is
[Noble gas] (n-1)d1-10ns1-2
For 3d series
[Ar] 3d1-10 4s1-2 (Scandium to Zinc)
Exceptional Configurations:
Cr: [Ar] 3d5 4s1
Cu: [Ar] 3d10 4s1
For 4d transition series
[Kr] 4d 1-10 5s1-2 (Ytterbium to Cadmium)
Exceptional Configurations:
Nb: [Kr] 4d4 5s1
Mo: [Kr] 4d5 5s1
Tc: [Kr] 4d6 5s1
Ru: [Kr] 4d7 5s1
Rh: [kr] 4d8 5s1
Pd: [Kr] 4d10 5s0
Ag: [Kr] 4d10 5s1
Why are Zinc, Cadmium and Mercury not regarded as transition metals?
Notice an intriguing aspect of the periodic table: zinc (Zn), cadmium (Cd), and mercury (Hg) are often excluded from the category of transition metals.
While they are located within the d block, their classification is rooted in their unique electron configurations and chemical properties. Transition metals are defined by their ability to form variable oxidation states and to create colored compounds, a behavior closely associated with the presence of unpaired d electrons.
However, zinc, cadmium, and mercury display a full complement of d electrons in their outer shells, making them distinct from their transition metal counterparts.
For instance, zinc has an electron configuration of [Ar] 3d10 4s2. In this configuration, all ten d electrons are paired, resulting in no variable oxidation states, as zinc typically exhibits a +2 oxidation state.
This pattern continues for cadmium and mercury, both of which maintain a completely filled d subshell, which inhibits the characteristic attributes of traditional transition metals.
Additionally, another key characteristic of transition metals is their ability to form complex ions with variable coordination numbers. Zinc, cadmium, and mercury typically form simple ions rather than complex ones and often do not exhibit the colorful compounds associated with transition metals.
Furthermore, due to the stability and filled nature of their d orbitals, these elements generally exhibit less reactivity compared to their transition metal neighbors.
In conclusion, understanding d block electron configuration charts is essential for anyone looking to grasp the intricacies of chemistry and atomic structure.
We hope this blog post has provided you with valuable insights and a clear roadmap for mastering these configurations. Whether you’re a student preparing for exams, a professional refreshing your knowledge, or simply a curious learner, utilizing tools like myetutors can enhance your understanding and help you navigate through complex topics with ease.
Remember, mastering electron configurations opens up a world of possibilities in the study of elements and their behaviors. If you have any questions or want to delve deeper into this subject, we’re just a click away at myetutors. Thank you for reading, and happy studying!
