Curriculum
- 12 Sections
- 69 Lessons
- 100 Hours
- Chapter 1 (Solid State)4
- Chapter 2 (Solutions)10
- 2.0Types of solutions20 Minutes
- 2.1Ways of expressing concentrations 120 Minutes
- 2.2Ways of expressing concentrations 220 Minutes
- 2.3Henry’s Law and applications 120 Minutes
- 2.4Raoult’s Law and applications 220 Minutes
- 2.5Colligative property 110 Minutes
- 2.6Colligative property 215 Minutes
- 2.7Colligative property 315 Minutes
- 2.8Colligative property 415 Minutes
- 2.9Abnormal Molecular Mass15 Minutes
- Chapter 3 (Electro Chemistry)8
- 3.1Electrolytic conductivity 120 Minutes
- 3.2Debye Huckel limiting law15 Minutes
- 3.3Electrochemical Cell20 Minutes
- 3.4Cell potential in electrochemistry20 Minutes
- 3.5How to use Nernst equation20 Minutes
- 3.6Gibbs free energy in electrochemistry20 Minutes
- 3.7Understand Faradays Laws of Electrolysis20 Minutes
- 3.8Fundamentals of commercial batteries20 Minutes
- Chapter 4 (Chemical Kinetics)6
- 4.1How to calculate rate of reaction20 Minutes
- 4.2How to calculate reaction law and orders20 Minutes
- 4.3Initial rate method know it all faster15 Minutes
- 4.4How to use integral method in kinetics 120 Minutes
- 4.5How to use integral method in kinetics 215 Minutes
- 4.6How Collision Theory Explains Chemical Reactions20 Minutes
- Chapter 8 (d-block Elements)7
- 5.1d block electron configuration charts10 Minutes
- 5.2Trend across d block 1 atomic radii10 Minutes
- 5.3Trend across d block 2 melting point10 Minutes
- 5.4Trend across d block 3 Ionization energy10 Minutes
- 5.5Trend across d block 4 Oxidation state10 Minutes
- 5.6Trend in d block 5 Electrode Potential10 Minutes
- 5.7Trend in d block metal properties
- Chapter 9 (Co-ordinate Compounds)6
- Chapter 10 (Haloalkanes Haloarenes)7
- Chapter 11 (Alcohols, Phenols Ethers)7
- Chapter 12 (Aldehyde Ketones)4
- Chapter 12 (Carboxylic acid, Derivatives)3
- Chapter 13 (Amines)3
- Chapter 14 (Biomolecules)4
Debye Huckel limiting law
Deby Huckel limiting law equation
Table of Contents
Introduction
In the world of physical chemistry, understanding ionic behavior is crucial for deciphering the intricacies of solutions, especially when it comes to electrolytes.
The Debye Huckel limiting law equation provides a foundational framework for studying the relationship between conductivity and electrolyte concentration, effectively distinguishing between strong and weak electrolytes.
Whether you’re a student delving into the fascinating realm of ionic interactions or a seasoned researcher seeking to refine your knowledge, this blog post will unravel the complexities of the Debye Huckel limiting law equation.
We will explore its significance, assumptions, and real-world applications, offering valuable insights that will enhance your comprehension of conductivity variations in different electrolytic environments.
Join us as we demystify this essential principle and empower you to navigate your chemistry studies with confidence!
Variation in Conductivity with dilution
The variation in specific conductivity with dilution is a fundamental concept in the study of electrolytes, especially when examining the Debye Huckel limiting law equation.
This law provides valuable insights into how strong and weak electrolytes behave as they are diluted, impacting their conductivity.
In essence, specific conductivity (κ) refers to the ability of an electrolyte solution to conduct electricity, which depends on the concentration of ions present in the solution.
As we dilute an electrolyte—whether it’s a strong electrolyte like sodium chloride or a weak electrolyte like acetic acid—two main phenomena occur.
Firstly, the absolute number of ions in the solution decreases with dilution, leading to a lower concentration of charge carriers available to conduct electric current. This results in typically lower conductivity values at higher dilutions.
However, what’s fascinating is that individual ions become more dissociated as the solution dilutes, particularly in the case of weak electrolytes. For weak electrolytes, the degree of dissociation increases, which means that the effective charge carriers can still contribute to conductivity despite the lower overall concentration.
As per the Debye Huckel limiting law equation, we can explain this behavior mathematically. The limiting law predicts that conductivity will approach a constant value as dilution continues, represented by the specific conductivity of the ions themselves.
For strong electrolytes, this reflects complete dissociation, while for weak electrolytes, it illustrates how dissociation is influenced by dilution, ultimately leading to a complex interplay between ion concentration and effective conductivity.
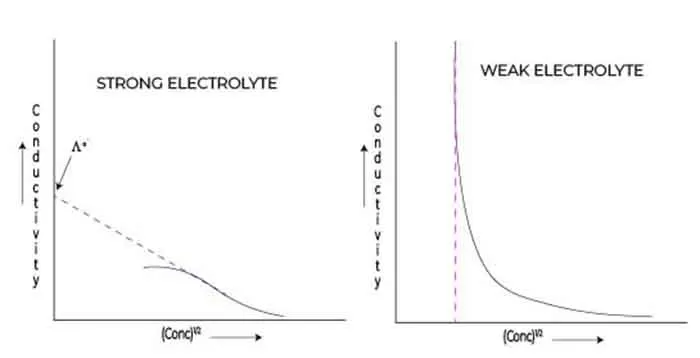
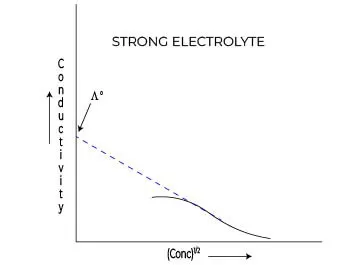
Variation in conductivity for strong electrolytes
When it comes to understanding the behavior of electrolytes in solution, the Debye Huckel limiting law provides a foundational framework for predicting how conductivity varies, particularly for strong electrolytes.
Strong electrolytes, which fully dissociate into their constituent ions in solution, exhibit unique characteristics that set them apart from their weak counterparts. The conductivity of a strong electrolyte can be primarily attributed to the concentration and mobility of the ions produced upon dissociation.
According to the Debye Huckel limiting law, the molar conductivity (ΛM) of a strong electrolyte can be expressed as a function of ion concentration, highlighting that as the ionic strength of the solution increases, the interactions between the ions become more significant.
This phenomenon is essential for understanding how strong electrolytes behave in various conditions. In dilute solutions, the conductivity is relatively straightforward to measure, as the ions are not heavily influenced by each other.
However, as the concentration increases, the conductivity deviates from ideal behavior due to increased electrostatic interactions among ions. This results in a decrease in the mobility of the ions, which the Debye Huckel limiting law equation accounts for by introducing corrections to the theoretical values of conductivity.
The equation for strong electrolytes typically highlights the relationship between conductivity and concentration, demonstrating that while conductivity increases with the addition of electrolyte, it does so at a decreasing rate due to these ionic interactions.
This nuanced behavior is critical for various applications, including the formulation of batteries, the design of chemical processes, and the study of biophysical systems.
By understanding these principles, we can better harness the properties of strong electrolytes to serve a multitude of scientific and industrial purposes.
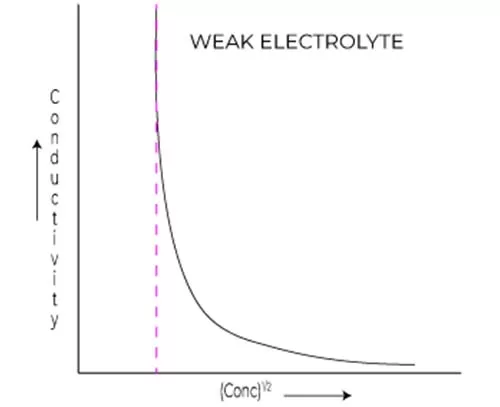
Variation in conductivity for weak electrolytes
When it comes to understanding the behavior of weak electrolytes, the Debye Huckel limiting law equation becomes an invaluable tool in the realm of physical chemistry.
Weak electrolytes, unlike their strong counterparts, do not fully dissociate in solution, which significantly affects their conductivity. This nuance introduces complexity when trying to predict how the conductivity of these electrolytes will change with concentration.
The Debye Huckel limiting law equation provides a framework for explaining this variation by considering the interactions between ions in a solution. As the concentration of a weak electrolyte increases, the degree of dissociation may not escalate linearly due to the influence of ion-ion interactions.
In essence, these interactions tend to shield ions from one another, thus impacting their ability to carry electrical current. As a result, the conductivity of weak electrolytes shows a distinct non-linear relationship with concentration, diverging from the predictions for strong electrolytes.
In practical terms, this means that while adding more of a weak electrolyte to a solution may intuitively suggest increased conductivity, the reality is more nuanced. The increase in conductivity will typically be less than what might be expected due to the reduced mobility of ions stemming from increased ionic strength.
The Debye Huckel limiting law comes into play by allowing us to model this conductivity variation. By incorporating the ionic activities into the equation, we can better approximate how a weak electrolyte behaves under different concentration scenarios.
This not only aids in calculations but also enhances our understanding of the underlying principles governing electrolyte solutions, bridging theoretical chemistry with practical application in fields ranging from pharmaceuticals to environmental science.
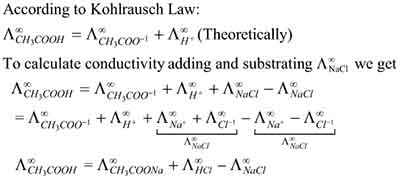
Kohlrausch law
The Kohlrausch Law provides a compelling framework for understanding the independent migration of ions in electrolytic solutions, particularly in the context of strong and weak electrolytes. Named after the pioneering work of chemist Wolfgang Kohlrausch, this law articulates how ions in solution move independently based on their individual mobilities, significantly impacting the overall conductivity of the electrolyte solution.
When applying the Kohlrausch Law, it’s essential to recognize that each ion contributes uniquely to the solution’s conductivity, depending on its charge and size. In a strong electrolyte, where dissociation into ions is complete, each ion migrates independently under the influence of an electric field.
This independent mobility means that the conductivity can be determined by the sum of the contributions from each ion type present. For example, in a sodium chloride (NaCl) solution, both sodium (Na⁺) and chloride (Cl⁻) ions migrate towards their respective electrodes, effectively enhancing the conductivity of the solution through their individual contributions.
In contrast, weak electrolytes, which partially dissociate in solution, introduce a layer of complexity. The degree of dissociation significantly affects the concentration of free ions, thereby altering the overall conductivity.
For weak electrolytes, the Kohlrausch Law still applies, but the contribution of each ion is weighed against the degree of dissociation. This results in a more nuanced understanding of how ion mobility and concentration interplay to affect conductivity. In effect, the Kohlrausch Law serves as a vital tool for chemists and students alike, providing insights into the behavior of ionic solutions.