Curriculum
- 12 Sections
- 69 Lessons
- 100 Hours
- Chapter 1 (Solid State)4
- Chapter 2 (Solutions)10
- 2.0Types of solutions20 Minutes
- 2.1Ways of expressing concentrations 120 Minutes
- 2.2Ways of expressing concentrations 220 Minutes
- 2.3Henry’s Law and applications 120 Minutes
- 2.4Raoult’s Law and applications 220 Minutes
- 2.5Colligative property 110 Minutes
- 2.6Colligative property 215 Minutes
- 2.7Colligative property 315 Minutes
- 2.8Colligative property 415 Minutes
- 2.9Abnormal Molecular Mass15 Minutes
- Chapter 3 (Electro Chemistry)8
- 3.1Electrolytic conductivity 120 Minutes
- 3.2Debye Huckel limiting law15 Minutes
- 3.3Electrochemical Cell20 Minutes
- 3.4Cell potential in electrochemistry20 Minutes
- 3.5How to use Nernst equation20 Minutes
- 3.6Gibbs free energy in electrochemistry20 Minutes
- 3.7Understand Faradays Laws of Electrolysis20 Minutes
- 3.8Fundamentals of commercial batteries20 Minutes
- Chapter 4 (Chemical Kinetics)6
- 4.1How to calculate rate of reaction20 Minutes
- 4.2How to calculate reaction law and orders20 Minutes
- 4.3Initial rate method know it all faster15 Minutes
- 4.4How to use integral method in kinetics 120 Minutes
- 4.5How to use integral method in kinetics 215 Minutes
- 4.6How Collision Theory Explains Chemical Reactions20 Minutes
- Chapter 8 (d-block Elements)7
- 5.1d block electron configuration charts10 Minutes
- 5.2Trend across d block 1 atomic radii10 Minutes
- 5.3Trend across d block 2 melting point10 Minutes
- 5.4Trend across d block 3 Ionization energy10 Minutes
- 5.5Trend across d block 4 Oxidation state10 Minutes
- 5.6Trend in d block 5 Electrode Potential10 Minutes
- 5.7Trend in d block metal properties
- Chapter 9 (Co-ordinate Compounds)6
- Chapter 10 (Haloalkanes Haloarenes)7
- Chapter 11 (Alcohols, Phenols Ethers)7
- Chapter 12 (Aldehyde Ketones)4
- Chapter 12 (Carboxylic acid, Derivatives)3
- Chapter 13 (Amines)3
- Chapter 14 (Biomolecules)4
How to calculate rate of reaction
How to calculate the rate of reaction
Table of Contents
How to calculate rate of reaction: Introduction
Understanding the rate of chemical reactions is a cornerstone of chemical kinetics, and mastering this concept can open doors to deeper insights in both academic and practical applications. Among the various techniques available, the graphical method stands out as a powerful tool to visualize and calculate reaction rates with clarity and precision.
Whether you’re a student navigating the complexities of chemistry or a professional seeking to enhance your problem-solving skills, grasping how to represent chemical data visually can transform your approach to reaction kinetics.
In this blog post, we will explore the fundamental principles of calculating the rate of chemical reactions through graphical methods, providing you with step-by-step guidance and practical tips to ensure your success in this essential area of study. With resources from myetutors at your disposal, you’ll be well-equipped to tackle these concepts with confidence!
Types of Chemical Reactions
In the realm of chemical kinetics, understanding the types of reactions is crucial for accurately calculating the rate of a chemical reaction using graphical methods. Chemical reactions can be categorized into three distinct types based on their rates:
- Instantaneous
- Moderate
- Fast reactions
Instantaneous Reactions:
The reactions which occur in a fraction of a second, often resulting in immediate visible changes. These reactions typically involve very short-lived intermediates or highly reactive species, making them almost instantaneous from a macroscopic viewpoint. A classic example is the combustion of hydrogen gas with oxygen to form water; this reaction happens so swiftly that the rate can be challenging to measure using standard methods.
In a graphical representation, the reaction progress would shoot up dramatically at the onset, illustrating a steep slope on the graph.
Moderate Reactions
The reactions are characterized by rates that are neither too fast nor too slow, making them easier to analyze over a reasonable period. These types of reactions take place over several minutes to hours and can involve several steps.
A common example of a moderate reaction is the esterification process, where an acid reacts with an alcohol to form an ester and water. When graphed, the data points would depict a gradual incline, indicating a steady but not overwhelming rate of change as the reaction progresses toward completion.
Fast Reactions:
These reactions as the name suggests, occur at rates that are significantly higher than those of moderate reactions but not as instantaneous as the first category. These reactions can manifest in just a few seconds to several minutes. An example would be the reaction between sodium and water, where effervescence and heat are produced rapidly. The graphical representation of a fast reaction shows a sharp rise but maintains a recognizable curve, allowing scientists to extract rate data and observe how quickly reactants are converted to products.
Graphical Method
In the study of chemical kinetics, one of the most insightful ways to understand the rate of a chemical reaction is through graphical analysis.
The graphical method for determining the rate of reaction provides a visual representation that simplifies the interpretation of data and reveals trends that may not be immediately apparent from raw numbers.
To start, you can plot concentration versus time on a graph. For many reactions, the relationship between the concentration of reactants or products and time can be linear, exponential, or follow some other mathematical relationship
Hoe to calculate rate of reaction with respect to reactants
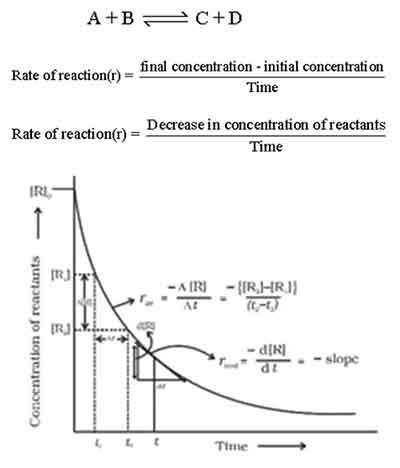
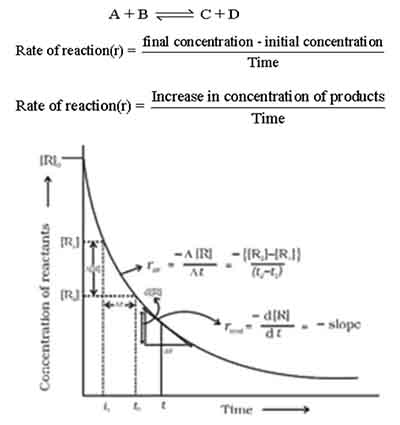
In the realm of chemical kinetics, understanding the rate of reaction in relation to the formation of products is pivotal for analyzing reaction dynamics.
The graphical method provides a clear and visual representation of how product concentration changes over time, allowing for a deeper insight into the nature of the reaction. To determine the rate of reaction with respect to products using the graphical method, you would typically begin by conducting the experiment and carefully measuring the concentration of products at various time intervals.
These measurements are then plotted on a graph, where the x-axis represents time and the y-axis signifies the concentration of the product. When you observe the resulting graph, you’ll likely notice that the concentration of products will increase over time as the reaction proceeds.
The steepness of the curve at any given point indicates the rate of reaction: a steeper slope signifies a faster reaction rate, while a gentler slope indicates a slower rate.
To accurately calculate the rate at a specific time, you can draw a tangent line at that point on the curve. The slope of this tangent will provide you with the instantaneous rate of reaction concerning the product.
This method not only allows you to gauge how quickly products are formed but also offers a visual representation of how the reaction progresses. In summary, utilizing the graphical method to analyze the rate of reaction with respect to products is an excellent approach for anyone looking to delve deeper into chemical kinetics.
It highlights the relationship between time and product formation, making it easier to understand the nuances of reaction dynamics.
Hoe to calculate rate of reaction with respect to products
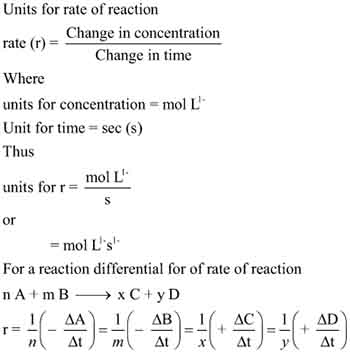
Units for rate of reaction
Understanding the units for the rate of reaction is vital for clear and meaningful analysis. The rate of a chemical reaction measures how quickly reactants turn into products over time. While the concept may seem straightforward, the units used to express this rate can vary based on the specific reaction dynamics. Typically, the rate of reaction is expressed in terms of concentration change per unit time.
For reactions involving gas or liquid concentrations, you’ll often see the unit of moles per liter (mol/L or M) as it reflects the molarity of a substance. This means that if a reactant concentration decreases by 0.1 M over a period of 10 minutes, the reaction rate can be calculated as 0.01 M/min, providing clarity on how swiftly the reactant is consumed.
For gaseous reactions, the units can also be expressed in terms of pressure change over time, such as atmospheres per second (atm/s). In a situation where gas is produced or consumed, measuring the change in pressure can offer a reliable indication of reaction progress.
It’s important to note that when you’re analyzing more complex reactions, like those involving multiple reactants or products, the rate of reaction could involve each component’s concentration change, leading to an overall reaction rate expressed in a more elaborate unit system.
Thus, keeping your units consistent and applicable to the specific context allows for accurate calculations when you move on to graphically representing your data and interpreting the results.
In conclusion, understanding how to calculate the rate of chemical reaction using graphical methods in chemical kinetics is a crucial skill for students and professionals alike. By applying the concepts we’ve discussed, including plotting concentration versus time and interpreting the slope of your graph, you can gain valuable insights into reaction dynamics and mechanisms.
With practice, you’ll find that these graphical methods not only enhance your analytical skills but also deepen your appreciation for the intricate behaviors of chemical reactions.
We encourage you to explore these techniques further with the help of myetutors, where expert guidance and resources are readily available to support your learning journey. Thank you for reading, and we look forward to seeing you excel in your studies!
