How to calculate reaction law and orders
How to calculate reaction law and orders
Table of Contents
Introduction
Understanding How to Calculate reaction law and orders in chemical kinetics is essential for any aspiring chemist, as it delves into the intricate dance of molecules during a reaction and the factors that influence their behavior. One of the fundamental challenges in this field is calculating reaction laws and orders, which can seem daunting at first.
However, mastering these concepts is key to predicting how chemical reactions unfold over time. In this blog post, we will break down the complexities of reaction law and order calculations, simplifying the process into manageable steps. Whether you’re a student striving to excel in your chemistry studies or an educator looking for effective teaching strategies, this guide will equip you with the tools needed to navigate the nuances of reaction kinetics with confidence and clarity,
Specific rate constant
In the realm of chemical kinetics, understanding how to Calculate reaction law and orders, first important concept is specific rate constant. It is crucial for grasping how reactions proceed over time. Often denoted as “k”, the specific rate constant is a unique value that reflects the inherent speed of a reaction at a given temperature.
It is essential to recognize that the specific rate constant is not a universal figure; rather, it varies depending on the type of reaction and its order. For a reaction represented by the generic equation:
A → B
where A and B are the reactant and product respectively, the specific rate constant helps quantify how the concentration of A decreases over time. This is particularly pertinent for determining the order of the reaction, as the rate law expresses the relationship between the rate of a reaction and the concentration of its reactants.

How to Calculate reaction law and orders, for instance, in a first-order reaction, the rate equation can be expressed as:
r = k[A]1
first order reaction n =1
Here, the concentration of reactant A is raised to the power of one, indicating a linear relationship where the rate of reaction is directly proportional to the concentration.
In contrast, a second-order reaction may take the form:
r= k[A]2
This indicates that the rate is proportional to the square of the concentration of A, representing a more complex interplay between concentration and reaction rate.
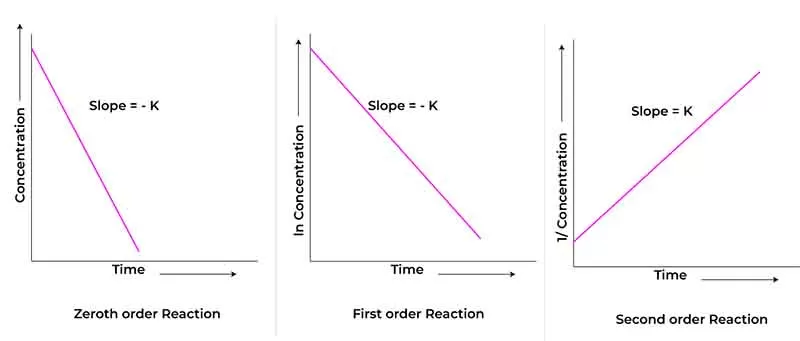
To determine the specific rate constant, you can plot the appropriate concentration data against time:
For a first-order reaction, a semi-logarithmic plot often reveals a straight line corresponding to the decay of concentration, allowing for straightforward calculation of k.
By precisely measuring conditions such as temperature and reactant concentrations, and analyzing the experimental data, one can discern the specific rate constant that characterizes a given reaction.
Units for Rate constant

Rate Law
In the realm of chemical kinetics how to Calculate reaction law and orders, the rate law is a pivotal equation that reveals the relationship between the rate of a chemical reaction and the concentration of its reactants. Understanding the rate law is essential for any student or professional studying reaction dynamics, and platforms like myetutors can provide invaluable assistance in grasping this critical concept.
The rate law is typically expressed in the form:
Rate = k[A]m[B]n
where
k denotes the rate constant,
[A] and [B] represent the molar concentrations of the reactants, and m and n signify the respective orders of the reaction with respect to each reactant.
The values of m and n are not always the same as the stoichiometric coefficients in the balanced equation; they must be determined experimentally.
To calculate the rate law, start by conducting an experiment to measure the reaction rate at various concentrations of the reactants. By systematically varying the concentration of one reactant while keeping the others constant, you can observe how the rate changes accordingly. This method enables you to determine the order of the reaction with respect to each reactant by using the initial rates method or integrated rate laws, depending on the level of complexity of the reaction.
Once the orders are established, you can plug them into the rate law equation, allowing you to predict how changes in concentrations will affect the overall reaction rate. This understanding is not just theoretical; it holds practical importance in fields ranging from pharmaceuticals to environmental science.
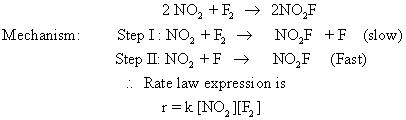
Order of Reaction
How to Calculate reaction law and orders in chemical kinetics, understanding the order of a reaction is crucial for predicting how the concentration of reactants will influence the speed of a chemical reaction. The order of reaction refers to the power to which the concentration of a reactant is raised in the rate law equation, reflecting the relationship between the concentration of reactants and the rate at which products are formed.
To determine the order of a reaction, chemists typically analyze experimental data, often gathered through various methods such as initial rate experiments or integrated rate laws. For instance, if a reaction between two reactants A and B is observed, and the rate of the reaction is found to be directly proportional to the concentration of A raised to the second power and to the first power of B,
In this case, the reaction is said to be second order with respect to A, first order with respect to B, and overall third order when you sum the individual orders. This information is invaluable; it allows chemists to predict the behavior of the reaction under different conditions.
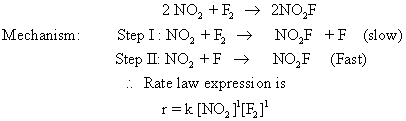
thus, the algebraic sum of exponential powers is 2.
In conclusion, understanding how to calculate reaction laws and orders is essential for anyone delving into the fascinating world of chemical kinetics. By mastering the principles outlined in this blog post from the importance of rate laws to the practical application of experimental data you can gain deeper insights into the mechanisms driving chemical reactions. Armed with this knowledge, you’ll not only enhance your academic pursuits but also amplify your analytical skills in real-world applications.
Whether you’re a budding chemist or a seasoned professional, utilizing resources like MyEtutors can help you refine these concepts even further. If you have any questions or want to deepen your understanding, feel free to reach out! Thank you for joining us on this journey through the intricacies of chemical kinetics.
