Curriculum
- 12 Sections
- 69 Lessons
- 100 Hours
- Chapter 1 (Solid State)4
- Chapter 2 (Solutions)10
- 2.0Types of solutions20 Minutes
- 2.1Ways of expressing concentrations 120 Minutes
- 2.2Ways of expressing concentrations 220 Minutes
- 2.3Henry’s Law and applications 120 Minutes
- 2.4Raoult’s Law and applications 220 Minutes
- 2.5Colligative property 110 Minutes
- 2.6Colligative property 215 Minutes
- 2.7Colligative property 315 Minutes
- 2.8Colligative property 415 Minutes
- 2.9Abnormal Molecular Mass15 Minutes
- Chapter 3 (Electro Chemistry)8
- 3.1Electrolytic conductivity 120 Minutes
- 3.2Debye Huckel limiting law15 Minutes
- 3.3Electrochemical Cell20 Minutes
- 3.4Cell potential in electrochemistry20 Minutes
- 3.5How to use Nernst equation20 Minutes
- 3.6Gibbs free energy in electrochemistry20 Minutes
- 3.7Understand Faradays Laws of Electrolysis20 Minutes
- 3.8Fundamentals of commercial batteries20 Minutes
- Chapter 4 (Chemical Kinetics)6
- 4.1How to calculate rate of reaction20 Minutes
- 4.2How to calculate reaction law and orders20 Minutes
- 4.3Initial rate method know it all faster15 Minutes
- 4.4How to use integral method in kinetics 120 Minutes
- 4.5How to use integral method in kinetics 215 Minutes
- 4.6How Collision Theory Explains Chemical Reactions20 Minutes
- Chapter 8 (d-block Elements)7
- 5.1d block electron configuration charts10 Minutes
- 5.2Trend across d block 1 atomic radii10 Minutes
- 5.3Trend across d block 2 melting point10 Minutes
- 5.4Trend across d block 3 Ionization energy10 Minutes
- 5.5Trend across d block 4 Oxidation state10 Minutes
- 5.6Trend in d block 5 Electrode Potential10 Minutes
- 5.7Trend in d block metal properties
- Chapter 9 (Co-ordinate Compounds)6
- Chapter 10 (Haloalkanes Haloarenes)7
- Chapter 11 (Alcohols, Phenols Ethers)7
- Chapter 12 (Aldehyde Ketones)4
- Chapter 12 (Carboxylic acid, Derivatives)3
- Chapter 13 (Amines)3
- Chapter 14 (Biomolecules)4
Trend across d block 2 melting point
Trend across d block 2 melting point
Table of Contents
Introduction
In the ever-evolving landscape of chemistry and material science, the trend across d block 2 melting point serves as a crucial indicator of a substance’s thermal properties and stability.
As we delve into the fascinating world of d block, understanding the trends that affect melting points becomes essential for researchers and enthusiasts alike.
In this blogpost, we will explore the intriguing nuances of melting point trends across various d block elements, shedding light on how elemental characteristics influence these critical thermal transitions.
Whether you’re a seasoned chemist or a curious learner, this blogpost trend across d block 2 melting point aims to provide valuable insights that will deepen your understanding of the science behind melting points to support your educational journey.
Melting Point
The trend across d block 2 melting point is a fundamental and captivating concept in the field of chemistry and materials science. Simply put, it is the temperature at which a solid substance transitions into a liquid state. This transformation occurs when the kinetic energy of the molecules within the solid increases, allowing them to break free from their rigid structure and begin to flow.
Understanding the trend across d block 2 melting point of a material is crucial for several reasons. It not only helps in determining its physical properties but also plays a vital role in various applications ranging from everyday cooking to advanced industrial processes. For example, when you heat a block of ice, it remains solid until it reaches 0°C (32°F), at which point it begins to melt and turn into water.
This specific temperature is the melting point of ice. Different substances have distinct melting points, which are influenced by their molecular composition and the strength of the bonds between their molecules.
For instance, metals typically have high melting points due to the robust metallic bonds present in their atomic structure, while many organic compounds may have relatively low melting points.
In practical applications, knowing the melting point assists scientists and engineers in material selection, quality control, and even the formulation of products.
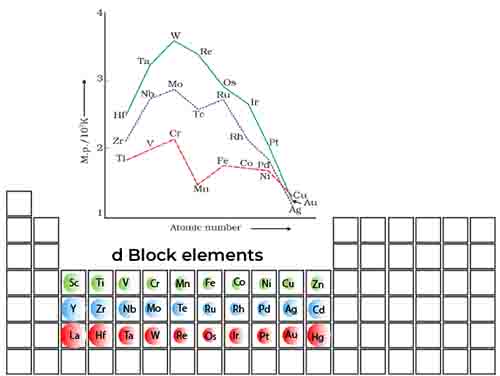
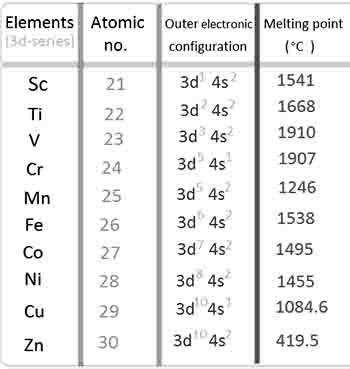
Trend in 3d series
Understanding the trend across d block 2 melting point the transition metals in the 3d series reveals fascinating insights into the elements’ physical properties and bonding characteristics.
As we navigate through the elements from scandium (Sc) to zinc (Zn), we initially observe an upward trajectory in melting points, peaking at Chromium (Cr), before witnessing a decline that continues through to copper (Cu) and zinc (Zn). This intriguing pattern can be attributed to the nature of interatomic metallic bonding, which is significantly influenced by the occupancy of d orbitals.
In the early transition metals, the d orbitals are only partially filled, resulting in a weaker shielding effect on the 4s electrons. As a consequence, the effective nuclear charge (Z effective) that is felt by the valence shell electrons becomes stronger as additional d electrons enter the system.
This increase in Z effective enhances the overall attraction between the positively charged nucleus and its surrounding electrons, thereby fortifying the interatomic metallic bonding. As we progress through the series, the d orbitals become more filled, especially after Iron (Fe), which leads to a reduction in the effective nuclear charge felt by the outer electrons. This shift causes a decrease in the strength of metallic bonding, resulting in lower melting points for elements like nickel (Ni) and copper (Cu).
The interplay between d orbital occupancy and effective nuclear charge plays a vital role in determining the melting points of these metals, illustrating the complex yet beautiful relationships that govern material properties in the 3d series.
By comprehending these trends across d block 2 melting point, students and enthusiasts alike can better appreciate the nuanced behavior of transition metals and their practical applications in various industrial processes and chemical reactions.
Trend in 4d and 5d series
Understanding the trend across d block 2 melting point for the 4d (from Y to Cd) and 5d (from La to Hg) series of transition metals provides fascinating insights into the complex world of interatomic interactions.
In both series, a notable trend emerges: the melting points initially increase and then decrease as we move across the period. This trend can be attributed to the intricate balance of interatomic metallic bonding that develops due to the filling of the d orbitals. As we progress through the 4d and 5d series, the number of d electrons increases, which typically enhances the strength of metallic bonds. The d orbitals begin to fill, and a significant interatomic metallic bonding occurs due to the increasing overlap of these orbitals.
However, when the d orbital occupancy is less than half-filled, an interesting phenomenon occurs. The d electrons fail to effectively shield the electrons in the outermost s orbitals—specifically, the 5s and 6s electrons. This results in a stronger effective nuclear charge (Z-effective) acting on the valence electrons. As a result, the valence shell electrons and those of surrounding atoms experience a stronger attractive force, leading to enhanced interatomic metallic bonding.
Initially, this causes a rise in melting points, as the atoms require more energy to overcome these strong attractive forces. However, as we continue to fill the d orbitals further and approach a half-filled structure, the bonding interactions change.
The increased electron-electron repulsion and the filling of the d subshell begin to negatively impact the overall metallic bonding, leading to a decrease in melting points. Thus, the melting point trend across the 4d and 5d series reflects a delicate interplay between atomic structure and bonding dynamics, revealing not just the characteristics of individual elements, but also the profound principles governing the behavior of materials at the atomic level.
