Curriculum
- 12 Sections
- 69 Lessons
- 100 Hours
- Chapter 1 (Solid State)4
- Chapter 2 (Solutions)10
- 2.0Types of solutions20 Minutes
- 2.1Ways of expressing concentrations 120 Minutes
- 2.2Ways of expressing concentrations 220 Minutes
- 2.3Henry’s Law and applications 120 Minutes
- 2.4Raoult’s Law and applications 220 Minutes
- 2.5Colligative property 110 Minutes
- 2.6Colligative property 215 Minutes
- 2.7Colligative property 315 Minutes
- 2.8Colligative property 415 Minutes
- 2.9Abnormal Molecular Mass15 Minutes
- Chapter 3 (Electro Chemistry)8
- 3.1Electrolytic conductivity 120 Minutes
- 3.2Debye Huckel limiting law15 Minutes
- 3.3Electrochemical Cell20 Minutes
- 3.4Cell potential in electrochemistry20 Minutes
- 3.5How to use Nernst equation20 Minutes
- 3.6Gibbs free energy in electrochemistry20 Minutes
- 3.7Understand Faradays Laws of Electrolysis20 Minutes
- 3.8Fundamentals of commercial batteries20 Minutes
- Chapter 4 (Chemical Kinetics)6
- 4.1How to calculate rate of reaction20 Minutes
- 4.2How to calculate reaction law and orders20 Minutes
- 4.3Initial rate method know it all faster15 Minutes
- 4.4How to use integral method in kinetics 120 Minutes
- 4.5How to use integral method in kinetics 215 Minutes
- 4.6How Collision Theory Explains Chemical Reactions20 Minutes
- Chapter 8 (d-block Elements)7
- 5.1d block electron configuration charts10 Minutes
- 5.2Trend across d block 1 atomic radii10 Minutes
- 5.3Trend across d block 2 melting point10 Minutes
- 5.4Trend across d block 3 Ionization energy10 Minutes
- 5.5Trend across d block 4 Oxidation state10 Minutes
- 5.6Trend in d block 5 Electrode Potential10 Minutes
- 5.7Trend in d block metal properties
- Chapter 9 (Co-ordinate Compounds)6
- Chapter 10 (Haloalkanes Haloarenes)7
- Chapter 11 (Alcohols, Phenols Ethers)7
- Chapter 12 (Aldehyde Ketones)4
- Chapter 12 (Carboxylic acid, Derivatives)3
- Chapter 13 (Amines)3
- Chapter 14 (Biomolecules)4
Trend across d block 3 Ionization energy
Trend across d block 3 Ionization energy
Table of Contents
Introduction
Understanding the Trend Across d Block 3 Ionization Energy in periodic table is crucial for anyone delving into the fascinating world of chemistry. One area that warrants particular attention is the behavior of ionization energy along the d-block elements.
As these transition metals exhibit unique properties and bonding characteristics, analyzing their ionization energy trends can unveil insightful aspects of their chemical reactivity and stability. In this blog post, we will explore the factors that influence ionization energy across the d-block, highlight key trends and anomalies, and uncover the implications of these patterns for both theoretical understanding and practical applications within the realm of chemistry.
Whether you’re a student looking to enhance your knowledge or a curious enthusiast eager to grasp the intricacies of elemental behavior, this exploration will provide a comprehensive overview and deepen your appreciation of the dynamic trend Across d Block 3 Ionization Energy in the periodic table.
Ionization Energy
Ionisation energy, often symbolized as IE, signifies a fundamental concept in the realm of chemistry and physics, playing a pivotal role in understanding how elements interact and behave on the atomic level. It represents the minimum amount of energy necessary to detach an electron from a neutral atom in its gaseous form, resulting in the formation of a positively charged ion.
This critical threshold of energy is not just a number; it encapsulates the inherent stability of an element’s electronic configuration and its propensity to engage in chemical reactions. When we delve into the specifics of ionization energy, we find that it is influenced by several factors, including atomic size, nuclear charge, and electron shielding.
Trend in 3d Series
The trend across d block 3 ionization energy of elements, which includes transition metals from scandium (Sc) to zinc (Zn), reveals some fascinating behaviors driven by the unique electron configurations of these elements. Initially, as we move from scandium to manganese, the ionization energy exhibits a gradual increase.
This trend across d block 3 ionization energy can be attributed to the behavior of the 4s and 3d electrons as they fill and interact within the atomic structure. In the early d-block elements particularly up until the 3d orbitals begin to half-fill the 4s electrons are relatively unshielded. With fewer electrons in the d orbitals, there is minimal repulsion from the d electrons, allowing the strong attractive force of the positively charged nucleus to dominate.
As a result, these 4s electrons experience an increasing nuclear charge, leading to enhanced ionization energies. However, upon reaching the half-filled state of the 3d orbitals, particularly in the case of chromium (Cr) and manganese (Mn), the situation changes. When the 3d orbitals contain five electrons, the stability of a half-filled d sublevel contributes to a decrease in the effective nuclear charge felt by the 4s electrons.
This shift is significant, as the incomplete d orbitals begin to provide a shielding effect, wherein the presence of additional 3d electrons reduces the overall attraction of the nucleus for the outermost 4s electrons. As we continue across the d block to the elements beyond manganese, ionization energy starts to decrease, particularly as the d orbitals become more than half-filled in elements like iron (Fe), cobalt (Co), and nickel (Ni). In these cases, the increased repulsion among electrons in both the 3d and 4s orbitals contributes to a lower ionization energy.
This trend across d block 3 ionization energy culminates with zinc (Zn), where the 3d orbitals are fully filled, leading to an even greater shielding effect that further reduces the energy required to remove the outermost electron.
Thus, the observed trend in ionization energy across the d-block elements is a result of intricate electron interactions, with the half-filled and fully filled configurations of the 3d orbitals playing pivotal roles in influencing the effective nuclear charge experienced by 4s electrons.
Understanding these nuances not only sheds light on the physical properties of these elements but also helps in predicting their chemical behavior in various reactions—insights that can be particularly valuable for students and researchers alike.
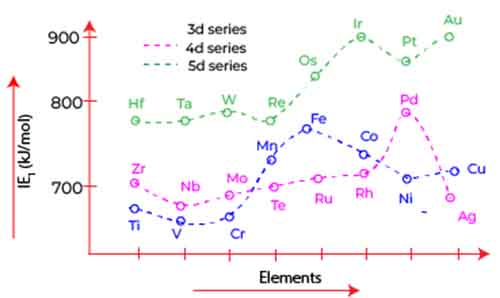
In conclusion, understanding the trend across d block 3 ionization energy among the 3d elements from scandium to zinc reveals intriguing insights into atomic structure and electron interactions. As we have discussed, the unique behavior of d orbitals significantly influences the attraction and repulsion experienced by valence electrons—particularly the 4s electrons.
Initially, as the d orbitals approach a half-filled state, the shielding effect remains minimal, allowing the nuclear charge to exert a stronger pull on the 4s electrons, resulting in higher ionization energy.
However, once the d orbitals become more than half filled, the increased shielding from the electrons in the 3d subshell leads to a decline in ionization energy. This transition encapsulates the delicate balance of electron distribution and its direct impact on elemental properties.
For more in-depth examination and tailored educational support, don’t hesitate to explore our offerings at Myetutors, where we are dedicated to helping you grasp complex concepts in chemistry and beyond. Thank you for engaging with us on this fascinating topic!
