As you embark on the journey of exploring the intricacies of chemistry, it is crucial to comprehend the various categories of solutions.
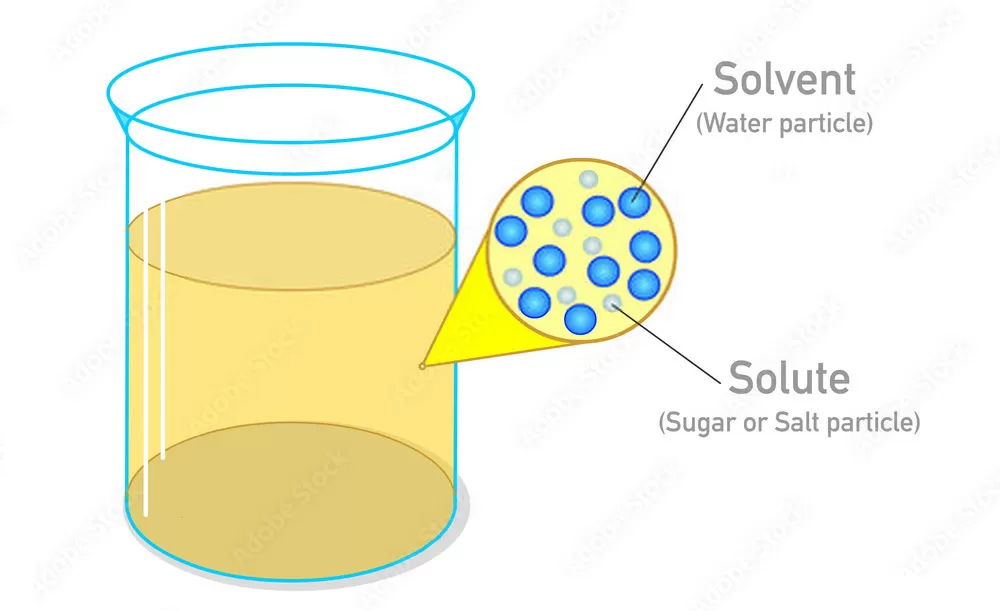
A solution is formed when a solute, one of the substances, is dissolved in a solvent, the other substance. Based on the properties of these components, solutions can be classified into two primary types:
homogeneous and heterogeneous solutions.
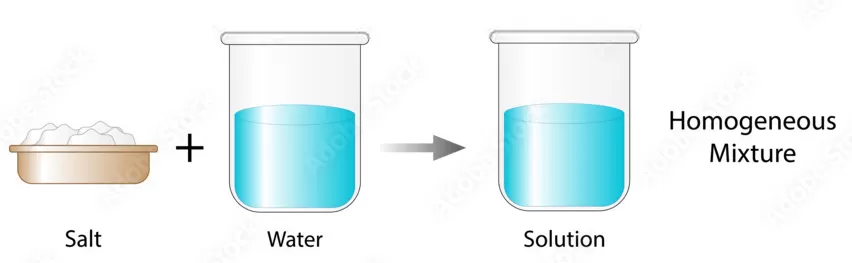
Homogeneous Solutions are characterized by a consistent and uniform composition and appearance throughout. This indicates that the solute particles are evenly dispersed in the solvent, making it impossible to distinguish between the two components with the naked eye. A perfect illustration of a homogeneous solution is saltwater. Upon adding salt (the solute) to water (the solvent), it dissolves entirely, forming a clear and uniform solution.
Additional instances encompass sweetener combined with tea or coffee and atmospheric air, consisting predominantly of various gases, namely nitrogen and oxygen. The consistent nature of these compounds results in them typically remaining unified and not separating into their individual elements when left to rest.
Diverse Solutions or Heterogeneous solution
Conversely, diverse solutions do not possess uniformity and can be isolated into distinctive components. In these solutions, the particles of solute are either not entirely dissolved or are of a larger size, making them discernible from the solvent.
A prevalent illustration of a diverse solution is the combination of oil and water. When stirred together, the two liquids do not smoothly combine; instead, the oil forms droplets that ascend to the surface, clearly visible against the water.
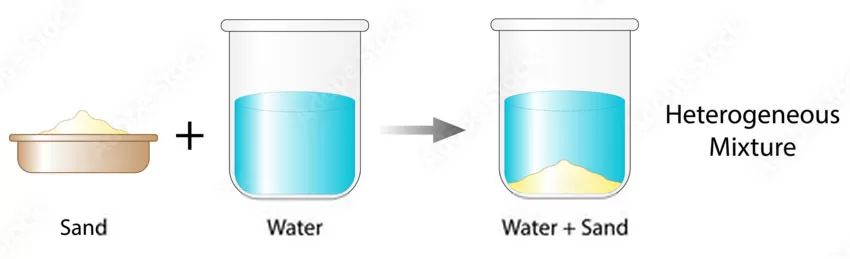
Another example is a mixture of sand and salt—although salt has the ability to dissolve in water, With the addition of sand, the mixture undergoes a transformation, separating into distinct layers and highlighting the unique properties of heterogeneous solutions.
By distinguishing between homogeneous and heterogeneous solutions, one can gain a deeper understanding of mixing behavior in the realm of chemistry.
Enlisting the help of online platforms such as myetutors can greatly aid in mastering these concepts, as they offer personalized tutoring sessions, interactive examples, and customized resources catered to your individual learning requirements.