Curriculum
- 12 Sections
- 69 Lessons
- 100 Hours
- Chapter 1 (Solid State)4
- Chapter 2 (Solutions)10
- 2.0Types of solutions20 Minutes
- 2.1Ways of expressing concentrations 120 Minutes
- 2.2Ways of expressing concentrations 220 Minutes
- 2.3Henry’s Law and applications 120 Minutes
- 2.4Raoult’s Law and applications 220 Minutes
- 2.5Colligative property 110 Minutes
- 2.6Colligative property 215 Minutes
- 2.7Colligative property 315 Minutes
- 2.8Colligative property 415 Minutes
- 2.9Abnormal Molecular Mass15 Minutes
- Chapter 3 (Electro Chemistry)8
- 3.1Electrolytic conductivity 120 Minutes
- 3.2Debye Huckel limiting law15 Minutes
- 3.3Electrochemical Cell20 Minutes
- 3.4Cell potential in electrochemistry20 Minutes
- 3.5How to use Nernst equation20 Minutes
- 3.6Gibbs free energy in electrochemistry20 Minutes
- 3.7Understand Faradays Laws of Electrolysis20 Minutes
- 3.8Fundamentals of commercial batteries20 Minutes
- Chapter 4 (Chemical Kinetics)6
- 4.1How to calculate rate of reaction20 Minutes
- 4.2How to calculate reaction law and orders20 Minutes
- 4.3Initial rate method know it all faster15 Minutes
- 4.4How to use integral method in kinetics 120 Minutes
- 4.5How to use integral method in kinetics 215 Minutes
- 4.6How Collision Theory Explains Chemical Reactions20 Minutes
- Chapter 8 (d-block Elements)7
- 5.1d block electron configuration charts10 Minutes
- 5.2Trend across d block 1 atomic radii10 Minutes
- 5.3Trend across d block 2 melting point10 Minutes
- 5.4Trend across d block 3 Ionization energy10 Minutes
- 5.5Trend across d block 4 Oxidation state10 Minutes
- 5.6Trend in d block 5 Electrode Potential10 Minutes
- 5.7Trend in d block metal properties
- Chapter 9 (Co-ordinate Compounds)6
- Chapter 10 (Haloalkanes Haloarenes)7
- Chapter 11 (Alcohols, Phenols Ethers)7
- Chapter 12 (Aldehyde Ketones)4
- Chapter 12 (Carboxylic acid, Derivatives)3
- Chapter 13 (Amines)3
- Chapter 14 (Biomolecules)4
Understand Faradays Laws of Electrolysis
Understand Faradays Laws of Electrolysis
Table of Contents
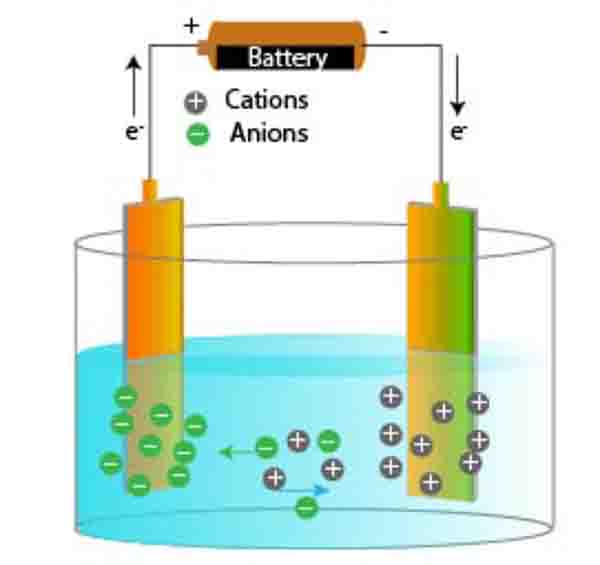
Introduction
Electrochemistry can often seem like a complex subject with its intricate theories and principles, but understanding its foundational concepts, such as Understand Faradays Laws of Electrolysis, is crucial for anyone diving into the world of electrolysis.
Faraday’s groundbreaking work not only laid the groundwork for modern electrochemistry but also empowers students and professionals alike to navigate the processes of electrolysis with confidence.
In this blog post, we’ll unravel the mysteries behind Faraday’s Laws, exploring how they apply to electrolytic solutions and everyday applications. Whether you’re a student seeking clarity for your studies or a practitioner wanting to enhance your knowledge, this comprehensive overview will enhance your understanding and effectiveness in carrying out electrolysis.
Join us as we demystify these essential electrochemical principles and their significance in real-world applications!
Understand Faradays Laws of Electrolysis: First Law
Understand Faradays Laws of Electrolysis, the principle of electrolysis is pivotal for anyone venturing into the field of electrochemistry, and it all begins with Faraday’s laws. The first of these, Faraday’s First Law of Electrolysis, lays the fundamental groundwork for our understanding of how electric current interacts with electrolytic solutions.
Formulated by the renowned scientist Michael Faraday in the 19th century, the First Law states that the amount of chemical change occurring at an electrode during electrolysis is directly proportional to the quantity of electric charge passed through the electrolyte. In simpler terms, this means that the greater the electric current flowing through the solution, the more significant the transformations that will take place at the electrodes.
Understand Faradays Laws of Electrolysis, visualize this, imagine conducting an electrolysis experiment using the myetutors platform. When you apply a certain voltage to your electrolytic solution, the flow of electric current causes ions to migrate towards the electrodes. According to Faraday’s First Law, if you double the current, you’ll double the amount of substance that gets deposited or liberated at the electrodes.
This concept is not only fundamental to laboratory applications but extends to various industries, from metal plating to electrorefining. Moreover, Faraday’s First Law helps define the principle of electrochemical equivalent, which is a constant that relates the quantity of substance altered at the electrodes to that of the electric charge.
Understand Faradays Laws of Electrolysis allows you to predict precisely how much material will be deposited during your experiments, thus enabling better control over the process. By mastering this important law, you’re well on your way to conducting successful electrolysis experiments and gaining a deeper insight into the fascinating world of electrochemistry.
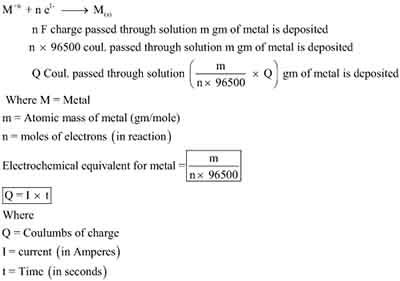
Understand Faradays Laws of Electrolysis: Second Law
The second law of electrolysis, formulated by Michael Faraday in the early 19th century, profoundly enhances our understanding of electrochemical processes. It states that the mass of an element that is deposited or liberated during electrolysis is directly proportional to the quantity of electric charge passed through the electrolyte.
Mathematically, it can be represented as:

This foundational principle reveals that the greater the charge passing through the solution, the higher the amount of substance that will be deposited or liberated.
Understand Faradays Laws of Electrolysis is vital when carrying out electrolysis with electrolytic solutions, as it allows chemists and practitioners to predict and control the outcomes of their experiments.
For example, if you are electrolyzing a copper sulfate solution to deposit copper onto an electrode, you can calculate the exact amount of copper you will obtain based on the current supplied and the time of electrolysis. This capability is especially important in industrial applications where materials must be accurately sourced and utilized.
Furthermore, the second law allows for the consideration of the electrochemical equivalent, which can be derived from the molar mass of the substance involved and its valency. With this knowledge, you can effectively choose the right conditions and parameters for your electrolysis process, ultimately leading to more efficient and predictable results.
By mastering Faraday’s second law, you’re not just following a scientific principle; you’re harnessing a tool that empowers your electrochemical endeavors.
In conclusion, understanding Faraday’s Laws of Electrolysis is essential for anyone delving into the world of electrochemistry and the practical applications of electrolysis.
These foundational principles not only illuminate the quantitative aspects of electrolysis but also enhance your ability to carry out experiments with precision and efficacy. With the insights gained from this blog post, you can confidently approach your electrochemical projects, whether in a laboratory setting or practical applications.
As you explore the fascinating interplay between electricity and chemical reactions, remember that myetutors is here to support your learning journey with expert guidance and resources.
Don’t hesitate to reach out if you have questions or need further assistance in mastering this intriguing subject!
