Friedel Craft acylation Reaction
- Posted by Chemistry instructor
- Categories General
- Date February 21, 2022
Friedel Craft acylation Reaction
Table of Contents

Introduction
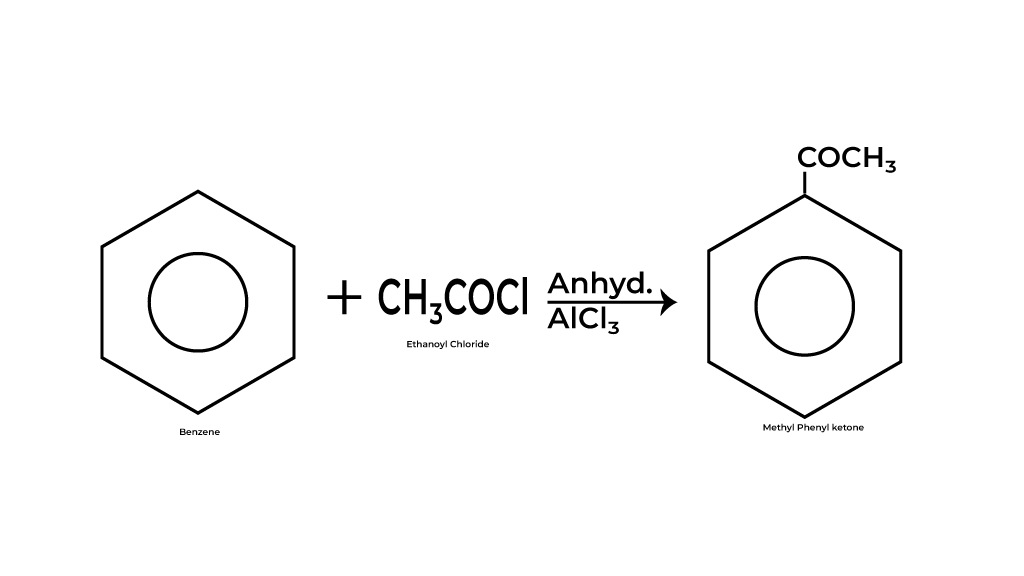
The world of organic chemistry can often seem daunting, filled with intricate reactions and complex mechanisms that challenge even the most seasoned students.
Among the myriad of reactions, Friedel Craft acylation Reaction stands out as a powerful method for introducing acyl groups to aromatic compounds, particularly benzene. This blog post will delve into the fascinating process of Friedel Craft acylation Reaction, exploring the formation of the crucial carbonium ion, the various resonating forms that stabilize this intermediate, and the overarching principles of electrophilic substitution reactions.
Whether you’re a chemistry enthusiast or a student aiming to master key concepts, this insightful exploration will illuminate the intricacies of these fundamental topics and enhance your understanding of aromatic chemistry. Join us as we unravel the mechanisms that make Friedel Craft acylation Reaction a cornerstone reaction in synthetic organic chemistry!
Reagents for Friedel Craft acylation Reaction
This transformative reaction hinges on a carefully selected set of reagents that are crucial for achieving successful acylation. Understanding these reagents is essential for students and practitioners alike, as they lay the foundation for both the mechanism and the efficiency of the reaction. The key reagent for Friedel Craft acylation Reaction is an acyl chloride (RCOCl), which serves as the acylating agent.
When paired with a strong Lewis acid catalyst, such as
- Anhydrous AlCl3
- Anhydrous FeCl3
- BF3
- TiCl3
- ZnCl2
- SnCl4
but most often aluminum chloride (AlCl₃) is used, this combination forms a highly reactive acylium ion (RCO⁺) that facilitates the electrophilic substitution reaction with benzene.
The role of aluminum chloride cannot be understated; it not only activates the acyl chloride by creating the acylium ion but also helps stabilize the intermediates throughout the reaction process. In some variations of the reaction, acid anhydrides can also be employed as acylating agents.
Acid anhydrides react similarly to acyl chlorides, generating acylium ions in the presence of a Lewis acid. This alternative offers a less corrosive method while still providing a highly effective means of acylation.
It’s worth noting that the presence of a proton source, such as water, can lead to hydrolysis and the formation of carboxylic acids unless carefully controlled; thus, a dry environment during the reaction is preferred.
By choosing the correct reagents and conditions, chemists can guide the Friedel Craft acylation Reaction to a successful conclusion, effectively creating a wide array of aromatic ketones.
In summary, the fundamental reagents used in the Friedel Craft acylation Reaction acyl chlorides or acid anhydrides in conjunction with a strong Lewis acid like aluminum chloride are crucial for facilitating this electrophilic substitution process.
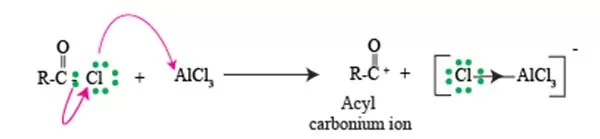
Formation of Acylium ion
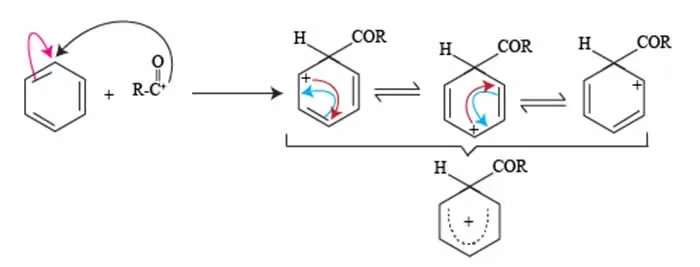
Resonance Stabilization of Sigma Complex
In Friedel Craft acylation Reaction. At the heart of this process lies the acylium ion, a key player that intricately influences the reactivity of benzene compounds.
When benzene engages in Friedel Crafts acylation, it first encounters an acylium ion, which is formed via the reaction of acyl chloride or anhydride with a Lewis acid, such as aluminum chloride. Once generated, this acylium ion (RCO^+) is characterized by a positively charged carbon atom, making it a powerful electrophile ready to engage with the delocalized electrons of the benzene ring.
The stability of the resulting carbonium ion (or sigma complex) is significantly enhanced through resonance stabilization. This phenomenon occurs when the positive charge of the carbonium ion is delocalized across the aromatic system, allowing for multiple resonance structures to coexist.
For instance, after the initial attack of the acylium ion on benzene, the structure can rearrange, distributing the positive charge among the carbon atoms of the benzene ring. This resonance effect not only stabilizes the intermediate but also facilitates the reestablishment of aromaticity once the reaction progresses.
The resulting stabilization lowers the energy barrier for the reaction, making the electrophilic substitution of benzene not only feasible but efficient in forming substituted aromatic compounds.
In summary, resonance stabilization of the benzene acylium ion is a vital aspect of the Friedel Craft acylation Reaction mechanism.
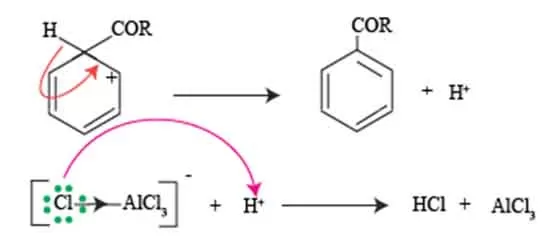
This ion, characterized by its positive charge and resonance structures, is pivotal in facilitating the electrophilic nature necessary for the substitution process. The acylium ion effectively acts as an electrophile, attacking the electron-rich π system of benzene and forming a new carbon-carbon bond.
However, the crucial aspect of this interaction lies in the reestablishment of benzene’s aromatic characteristics following the electrophilic attack. As the acylium ion binds to one of the carbon atoms in the benzene ring, it temporarily disrupts the aromaticity of the system. This disruption leads to the formation of a non-aromatic carbocation intermediate.
To restore its aromatic state, the intermediate undergoes a rearrangement where a proton is lost — a process that re-establishes the delocalized π system typical of aromatic compounds. The resulting product of the Friedel-Crafts acylation is an aromatic ketone, showcasing the successful reformation of aromaticity.
This restoration is critical, as it provides stability to the compound, allowing for further reactions or stability in subsequent chemical processes. Understanding this cycle of acylium ion formation, disruption of aromaticity, and eventual regeneration is vital for mastering electrophilic substitution reactions and their applications within organic synthesis.
You May Also Like
Become a Member
YouTube Channel
Telegram Channel
Tag:CBSE
ABOUT INSTRUCTOR
B.Sc (honors) Chemistry, M.Sc. (Organic Chemistry), Gold Medalist from Gujarat University Ahmedabad. Passionate educator, helping aspirants for IIT-JEE, NEET-UG, AP-Chemistry, IB-HL Chemistry, BIT-SAT, CBSE, and ICSE to achieve their ambitions
You may also like
Analysis of Ammonium Sulphate
Analysis of Ammonium Sulphate Ammonium Sulphate Chemical Composition: Ammonium sulphate, a colorless crystal solid with the chemical formula (NH₄)₂ SO₄, has a rich history and …
Cracking BITSAT: Expert Tips and Strategies to Ace the Exam “Cracking BITSAT: Expert Tips and Strategies to Ace the Exam” – A blog post that …
How to carry sulphonation of arenes
How to carry sulphonation of arenes Table of Contents Introduction How to carry sulphonation of benzene: Sulphonation of arenes, particularly benzene, is a fascinating reaction …
